- Case report
- Open access
- Published: 03 November 2022

Aplastic anemia: a new complication in the recent mysterious hepatitis outbreak among children worldwide: two case reports
- Ali Ghanei-Shahmirzadi 1 ,
- Hamid Reihani 1 ,
- Ali Abbasi-Kashkooli 1 ,
- Fereshteh Karbasian 2 ,
- Seyyed Bozorgmehr Hedayati 3 ,
- Mohammadreza Bordbar 3 ,
- Maryam Ataollahi 4 ,
- Seyed Mohsen Dehghani 4 &
- Bita Geramizadeh 5 , 6
Journal of Medical Case Reports volume 16 , Article number: 422 ( 2022 ) Cite this article
3578 Accesses
2 Citations
1 Altmetric
Metrics details
Recently, an unknown hepatitis outbreak among children has concerned many individuals worldwide. These cases are frequently reported, mainly from Europe and other countries. In this study, we present two similar patients, who, to the best of our knowledge, are the first cases reported in the Middle East (Shiraz, Fars Province, Iran). Unlike in similar cases reported up until 30 April 2022, our patients’ hepatitis eventually resulted in aplastic anemia.
Case presentation
In this study, we present cases of two Iranian boys aged 13 and 8 years with hepatitis of unknown origin who developed aplastic anemia in the course of hospitalization.
Conclusions
Hepatitis-associated aplastic anemia is a well-known immune-mediated form of aplastic anemia that we detected in our patients and treated with immunosuppressive therapy. One patient established a satisfactory response to the treatment, but unfortunately, the other was declared brain dead.
Peer Review reports
Introduction
Since November 2021, there have been reports of an unexplained hepatitis outbreak in several countries. Hepatitis can occur from various causes, including viral infections, autoimmune disorders, toxins, and medications [ 1 ]. In April 2022, 145 children with unknown severe hepatitis were reported in the UK, and nine other cases were reported in Alabama [ 2 , 3 ]. Furthermore, there have been similar reports from Denmark, Spain, Ireland, and Netherlands [ 3 ]. At the same time, two children were referred to our center in Shiraz, Iran with hepatitis manifestations, where further laboratory evaluations ruled out all regular causalities. Besides their hepatitis, we noticed pancytopenia in their complete blood count (CBC) test, which raised suspicion for probable bone marrow disorder. Therefore, a bone marrow biopsy was done, and the results were in favor of aplastic anemia.
Aplastic anemia is a rare but life-threatening condition in which bone marrow failure and hypocellularity result in progressive pancytopenia [ 4 , 5 ]. Aplastic anemia has been categorized into acquired and inherited types [ 6 ]. Acquired aplastic anemia is due to an abnormal immune response triggered by different environmental exposures, including drugs, toxins, and viral infections [ 6 ]. It appears that cytotoxic lymphocytes and type I cytokines have a role in autoimmune aplastic anemia, and evidence of low quantity and/or function of T-regulatory cells has been found [ 7 , 8 ]. Since our patients did not have any history of inherited aplastic anemia or any other risk factor for acquired aplastic anemia, we considered hepatitis to be the underlying cause of their aplastic anemia. Therefore, our diagnosis became hepatitis-associated aplastic anemia (HAAA). HAAA is a well-known immune-mediated form of acquired aplastic anemia in which an acute hepatitis episode results in acute or chronic bone marrow failure accompanied by pancytopenia [ 9 , 10 ]. HAAA was first mentioned in two cases in 1955 [ 11 , 12 ], but the number of reports reached more than 200 cases by 1975 [ 13 ]. Later on, it was documented in up to 2–5% of aplastic anemia cases in the West [ 14 , 15 ] and 4–10% of the cases in the Far East [ 16 ]. Consequently, owing to the extent of this new hepatitis outbreak among children and considering the rareness of having two patients in such a short period with this infrequent diagnosis in our center, we informed the healthcare providers of other aspects of this new outbreak in the hopes of achieving a faster diagnosis and, therefore, better outcome and prognosis for the patients. We present two cases of HAAA who were referred to our center.
A 13-year-old Iranian boy came to our pediatric emergency department, a referral center affiliated with Shiraz University of Medical Sciences, with the chief complaint of yellowish skin and two red spots on his right leg. Furthermore, his mother mentioned that he had nosebleeds for a week prior to the admission. He developed icterus 2 months before the admission, and after preliminary laboratory evaluations, which revealed elevated liver enzymes, he was diagnosed with hepatitis. One day before admission, his mother suddenly saw some petechiae-like lesions on his right leg, so she brought the boy to our center. We decided to check CBC and performed liver function tests (LFTs). His preadmission medications included folic acid 5 mg once a day and ursodeoxycholic acid 300 mg twice a day. Physicians had prescribed these drugs because of his previous hepatitis diagnosis. On our primary physical examination, his sclera appeared icteric. He had an ulcer on his lower lip. His lungs were clear, and his heart sounds were normal. On abdominal examination, his liver seemed to be slightly enlarged. He also had evidence of ecchymosis on his right leg.
Laboratory investigations revealed pancytopenia on the first CBC [white blood cell count (WBC) 900/µl, hemoglobin (HB) 7.8 g/dl, platelet count (PLT) 4000/µl]. His liver enzymes were elevated [aspartate transferase (AST) 68 U/L, alanine transaminase (ALT) 174 U/L, total bilirubin 1 mg/dL, direct bilirubin 0.29 mg/dL, gamma-glutamyl transpeptidase 26 U/L] as they had been over the past 2 months. Intending to find the cause of his hepatitis, we checked for common viral hepatitis causes, including hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), and Epstein–Barr virus (EBV), which were all negative. We checked anti-LKM antibody (Ab), anti-dsDNA Ab, cANCA, and pANCA to rule out the possibility of autoimmune disorders, though we did not find any of them to be positive. We also checked ceruloplasmin and serum copper levels to rule out Wilson’s disease, and neither was in favor of Wilson. We checked coronavirus disease 2019 (COVID-19) immunoglobulin (Ig)G and IgM, which were both negative. We also asked about his past drug history (including herbal drugs) and any potential toxin exposure, but we did not find that could have caused hepatitis. Moreover, we performed chromosome breakage test, which was negative, ruling out Fanconi anemia.
In terms of his pancytopenia, we performed bone marrow aspiration and biopsy. The results showed severe hypocellularity (approximately 15%), which was low for his age and suggestive of aplastic anemia. Pictures of bone marrow biopsy and aspiration are shown in Fig. 1 . Immunophenotyping by flow cytometry was done on his bone marrow sample, and there was no evidence of leukemia or lymphoma. Our treatment plan was immunosuppressive therapy (IST). The patient was started on equine antithymocyte globulin (ATG) (40 mg/kg/day for 4 days), prednisolone (0.5 mg/kg/day), and cyclosporine (10 mg/kg/day). After receiving treatment, his CBC stabilized (enough to discharge the patient from hospital with further follow-up scheduled) 10 days after IST initiation, and eventually, he was discharged in good condition.
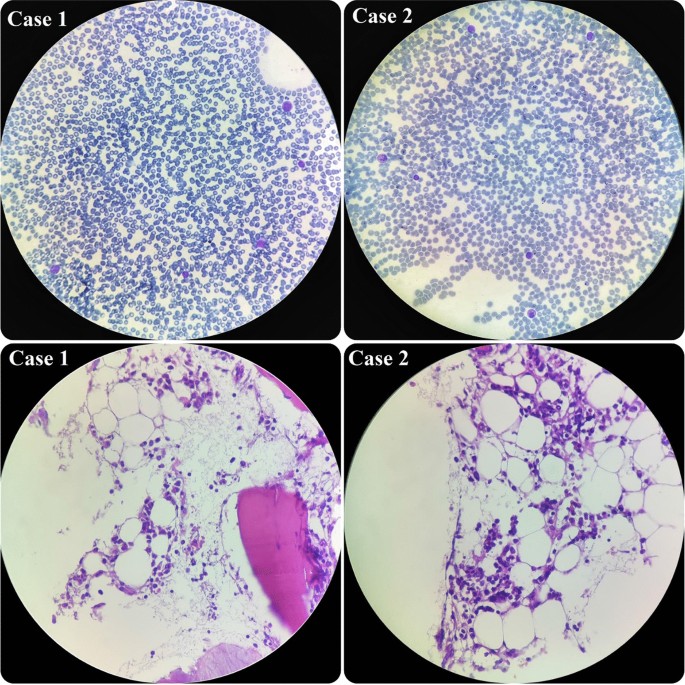
Hypocellular smear from bone marrow aspiration revealing a few scattered myeloid cells and lymphocytes. On bone marrow biopsy, which revealed hypocellularity for his age, intertrabecular marrow spaces were shown to have been replaced by fatty cells beside the presence of some nucleated cells, including lymphocytes, plasma cells, and erythroid cells
An 8-year-old Iranian boy was brought to our emergency department with the chief complaint of yellowish skin and abdominal distension. He was relatively well until 3 weeks prior to admission. His initial symptoms were anorexia, weakness, and fever, which led him to a medical center. Primary laboratory evaluations were done there, and he was diagnosed with fulminant hepatitis on the basis of elevated international normalized ratio (INR). He also had extremely high liver enzymes. At that medical center, he had received supportive care. Then, since our hospital is the referral liver transplant center in Iran, his parents brought him to our emergency department. On initial presentation, he was severely icteric. His abdomen was distended, and ascites and hepatomegaly were observed.
Our initial laboratory investigations showed severe elevation of his liver enzymes (AST 1615 U/L, ALT 1880 U/L, total bilirubin 47 mg/dL, direct bilirubin 22.8 mg/dL). At that time, he also had coagulation disorder [prothrombin time (PT) 21.1 seconds and INR 2.5]. Moreover, we detected anemia and mild leukopenia in his CBC, but his platelet count was normal (WBC 2900/µl, HB 8.3 g/dl, PLT 190,000/µl). We started searching for the cause of his hepatitis. After checking viral and immunological markers, the only noticeable item we found was a positive COVID-19 IgG Ab. Like the previous patient, he did not report any history of exposure to drugs (including herbal drugs) or hepatotoxic toxins. Moreover, Fanconi anemia was ruled out for the patient by negative chromosome breakage test.
During the admission course, his situation improved and his liver enzymes began to decrease, but he suddenly developed petechiae on his left hand. So, we immediately checked the CBC, which revealed severe pancytopenia (WBC 500/µl, HB 7.1g/dl, PLT 20,000/µl). His platelet count dropped drastically. Therefore, we planned for bone marrow aspiration and biopsy, and the result showed severe hypocellularity, approximately 20%, indicating aplastic anemia. Pictures of bone marrow aspiration and biopsy are shown in Fig. 1 .
Since his WBC count was very low, we could not consider a bone marrow transplant, and we decided to try immunosuppressive therapy for him, as the other patient had responded well to it. Unfortunately, after receiving the third dose of ATG, he had an episode of generalized tonic–clonic seizure, and he did not show any response to the treatment until that time. Hence, we stopped the chemotherapy and transferred him to the pediatric intensive care unit (ICU) ward. Unfortunately, he was declared brain dead, due to the low platelet number and coagulopathy. Our patients’ characteristics are presented in Table 1 .
Since January 2022, the world has faced an unknown hepatitis outbreak primarily reported in Europe and the USA, mainly in children under 10 years of age [ 2 , 17 ]. As we have been struggling with COVID 19 during the past 2 years, it is essential to clarify different aspects of this new challenge as soon as possible. Undoubtedly, the most crucial element is to determine the cause, as well as finding proper treatment and identifying the short- and long-term complications of this new hepatitis outbreak. Right now, the leading hypothesis about the source of this outbreak is an adenovirus [ 2 ]. However, it is not yet determined whether we are dealing with a new variant or whether the social distancing in these 2 years resulted in fewer exposures to the virus, making children’s naive immune system more susceptible to the previously existing types. It is noteworthy that adenovirus has been known previously to cause acute hepatitis in immunosuppressive children [ 18 ].
As of 29 April 2022, according to the World Health Organization (WHO) and UK Health Security Agency (UKHSA), there are at least 200 cases of acute hepatitis of unknown origin that have been reported from 11 countries [ 2 , 3 ]. Interestingly, 40 out of 53 patients in the UK and 9 out of 9 cases in Alabama that were tested for adenovirus had a positive result [ 2 , 17 ]. Moreover, the WHO stated that adenovirus had been detected in at least 74 cases [ 3 ]. Unfortunately, owing to the lack of laboratory equipment, we could not confirm adenovirus infection in our patients. However, our second case had a positive COVID-19 IgG test. It should be mentioned that, in the majority of confirmed cases, patients were not infected by the COVID-19 virus or vaccinated with COVID-19 vaccines at the time of developing hepatitis. Therefore, we can argue that there is no relation between this outbreak and COVID-19 infection. However, we should consider the high rate of COVID-19 over recent months, especially in children and particularly in England, which has more cases than other countries. This may result in the presentation of a new hepatitis type or mislead us because of its constant presence during this time. Therefore, it is better to perform further experiments to find more substantial evidence.
Severe aplastic anemia usually develops 2–3 months after acute hepatitis attack in patients with HAAA [ 9 ]. In our patients, the delay between hepatitis attack and aplastic anemia was close to 2 months as well. In both cases, we started immunosuppressive therapy as soon as the diagnosis of aplastic anemia was confirmed, which consisted of a combination of cyclosporine, ATG, and steroids. Previous studies have shown a 30–70% response to immunosuppressive therapy treatment in children with acquired aplastic anemia [ 19 , 20 , 21 ]. We tried the same regimen on our patients, and one of them benefited from this treatment. However, the other patient did not respond well owing to his poor condition, and eventually, he was declared brain dead. Although we used this particular IST regimen in our patients, we recommend to test other accepted regimens as well and compare and analyze the results to identify the best treatment.
Hepatitis-associated aplastic anemia (HAAA) is a well-known immune-mediated form of aplastic anemia that we detected in our patients. To the best of our knowledge, our study is the first to report the co-occurrence of aplastic anemia with the recent unknown outbreak of hepatitis among children. Therefore, we recommend being alert to hepatitis cases that develop signs and symptoms of pancytopenia, and performing further follow-ups for early diagnosis of potential aplastic anemia.
Availability of data and materials
Data of the patient can be requested from the authors. Do not hesitate to get in touch with the corresponding author if you are interested in these data.
Abbreviations
- Hepatitis-associated aplastic anemia
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869–81.
Article CAS PubMed Google Scholar
Increase in hepatitis (liver inflammation) cases in children under investigation https://www.gov.uk/government/news/increase-in-hepatitis-liver-inflammation-cases-in-children-under-investigation#full-publication-update-history : UK Health Security Agency; 2022.
Multi-Country—Acute, severe hepatitis of unknown origin in children https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 : WHO; 2022.
Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101(6):527–35.
Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19.
Article CAS PubMed PubMed Central Google Scholar
Altay D, Yılmaz E, Özcan A, Karakükçü M, Ünal E, Arslan D. Hepatitis-associated aplastic anemia in pediatric patients: single center experience. Transfus Apheres Sci. 2020;59(6): 102900.
Article Google Scholar
Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, et al . Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–6.
Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, et al . Intrinsic impairment of CD4+ CD25+ regulatory T cells in acquired aplastic anemia. Blood. 2012;120(8):1624–32.
Gonzalez-Casas R, Garcia-Buey L, Jones E, Gisbert J, Moreno-Otero R. Systematic review: hepatitis-associated aplastic anaemia—a syndrome associated with abnormal immunological function. Aliment Pharmacol Ther. 2009;30(5):436–43.
Rauff B, Idrees M, Shah SAR, Butt S, Butt AM, Ali L, et al . Hepatitis associated aplastic anemia: a review. Virol J. 2011;8(1):1–6.
Fomina L. K voprosu obizmenenii krovetvoreniia pri zabolevaniiakh pecheni. Sov Med. 1955;19(6):28–31.
CAS PubMed Google Scholar
Lorenz E, Quaiser K. Panmyelopathy following epidemic hepatitis. Wien Med Wochenschr. 1955;105(1):19–22.
Hagler L, Pastore RA, Bergin JJ, Wrensch MR. Aplastic anemia following viral hepatitis: report of two fatal cases and literature review. Medicine. 1975;54(2):139–64.
Böttiger L, Westerholm B. Aplastic anaemia: aplastic anaemia and infectious hepatitis. Acta Med Scand. 1972;192(1–6):323–6.
PubMed Google Scholar
Mary J, Baumelou E, Guiguet M. Epidemiology of aplastic anemia in France: a prospective multicentric study. Blood. 1990;75(8):1646–53.
Young NS, Issaragrasil S, Chieh CEW, Takaku F. Aplastic anaemia in the Orient. Br J Haematol. 1986;62(1):1–6.
Stubblefield W. Investigations of nine young children with adenovirus are underway www.alabamapublichealth.gov2022 .
Matoq A, Salahuddin A. Acute hepatitis and pancytopenia in healthy infant with adenovirus. Case Rep Pediatr. 2016;2016.
Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al . Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92(1):11–8.
Osugi Y, Yagasaki H, Sako M, Kosaka Y, Taga T, Ito T, et al . Antithymocyte globulin and cyclosporine for treatment of 44 children with hepatitis associated aplastic anemia. Haematologica. 2007;92(12):1687–90.
Jeong DC, Chung NG, Cho B, Zou Y, Ruan M, Takahashi Y, et al . Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664.
Download references
Acknowledgements
The authors are grateful to the patients and their parents for their participation in the current study.
Author information
Authors and affiliations.
Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Ali Ghanei-Shahmirzadi, Hamid Reihani & Ali Abbasi-Kashkooli
Department of Pediatric Gastroenterology, Shiraz University of Medical Sciences, Shiraz, Iran
Fereshteh Karbasian
Hematology research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Seyyed Bozorgmehr Hedayati & Mohammadreza Bordbar
Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Maryam Ataollahi & Seyed Mohsen Dehghani
Shiraz Transplant Research Center (STRC), Shiraz University of Medical Sciences, Shiraz, Iran
Bita Geramizadeh
Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
You can also search for this author in PubMed Google Scholar
Contributions
SD, FK, MA, BG, and MB designed the study and revised the manuscript. HR, AG, SH, and AA were in charge of collecting data and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Fereshteh Karbasian .
Ethics declarations
Ethics approval and consent to participate.
Our study has been reviewed and approved by the Medical Ethics Committee of Shiraz University of Medical Sciences.
Consent for publication
Written informed consent was obtained from the patients’ legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ghanei-Shahmirzadi, A., Reihani, H., Abbasi-Kashkooli, A. et al. Aplastic anemia: a new complication in the recent mysterious hepatitis outbreak among children worldwide: two case reports. J Med Case Reports 16 , 422 (2022). https://doi.org/10.1186/s13256-022-03542-0
Download citation
Received : 08 June 2022
Accepted : 25 July 2022
Published : 03 November 2022
DOI : https://doi.org/10.1186/s13256-022-03542-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aplastic anemia
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
Case Studies for Fellows
- American Society of Hematology
- Resources for Hematology Fellows
- Hematopoiesis Case Studies
- Three-year-old Boy With Pancytopenia
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
Case Study: Three-year-old Boy With Pancytopenia
A three-year-old boy with no significant medical history presents to his pediatrician with a two-month history of petechial rash and intermittent epistaxis. He has had a cough, has experienced rhinorrhea, and has been fussier than normal. No fevers, weight loss, or unusual fatigue were reported. He is tracking along his growth curves and is meeting his developmental milestones appropriately.
He is an only child, born full term at 38 weeks with no significant complications. There is no significant family medical history. His only recent medications include acetaminophen and diphenhydramine.
Physical examination shows that he is afebrile and has no dysmorphic features. Scattered petechiae noted on the patient's trunk and extremities, as well as a few ecchymoses on his bilateral lower extremities. There has been no hepatosplenomegaly or significant lymphadenopathy, and extremities are normal.
Laboratory Results
| Value | Reference Range | |
| Hemoglobin (g/dL) | 9.2 | 11.5-13.5 |
| Hematocrit (%) | 26.6 | 34.0-40.0 |
| Mean corpuscular volume (fL) | 88.7 | 75.0-87.0 |
| Leukocytes | 1.1 x 10 /L | 6.0-15.5 x 10 /L |
| Absolute neutrophil count | 207 x 10 /L | 1,500-7,770 x 10 /L |
| Platelets | 12 x 10 /L | 250-550 x 10 /L |
| Reticulocyte count | 29 x 10 /L | 18-100 x 10 /L |
The patient underwent a bone marrow biopsy and aspirate, which demonstrates marrow cellularity ranging from 5 to 20 percent cellularity (average 10%). No blasts or dysplastic features were identified. Karyotype analysis was unsuccessful. A diepoxybutane test was negative and whole-genome next-generation sequencing did not reveal any mutations.
Which of the following is the most appropriate management at this time?
- Monitor complete blood count every three months for worsening cytopenias with annual marrow evaluation
- Horse antithymocyte globulin and cyclosporine
- Intravenous immunoglobulin
- Eltrombopag
- Matched unrelated donor hematopoietic stem cell transplantation
Answer: B. Horse antithymocyte globulin and cyclosporine
This patient has severe aplastic anemia (SAA). SAA is most commonly immune-mediated and is defined as having bone marrow cellularity lower than 25 percent and two or more of the following 1 : peripheral blood neutrophil count lower than 0.5 × 10 9 /L; peripheral blood platelet count lower than 20 × 10 9 /L, and peripheral blood reticulocyte count lower than 20 × 10 9 /L.
There is no required duration of cytopenias to establish a diagnosis of aplastic anemia (AA).
AA is a diagnosis of exclusion. Diagnostic workup in pediatric patients often includes consideration of hematologic malignancies, viral etiologies such as parvovirus, and inherited bone marrow failure (BMF) syndromes. Inherited BMF syndromes encompass a wide range of disorders, and a thorough family history and patient physical examination may help target diagnostic evaluation. However, even in the absence of any notable features, diagnostic workup in patients with BMF should include chromosome breakage studies (diepoxybutane test) to rule out Fanconi anemia, telomere length studies to rule out dyskeratosis congenita, paroxysmal nocturnal hemoglobinuria test (fluorescently labeled inactive toxin aerolysin test), and pancreatic enzymes to evaluate for Shwachman-Diamond syndrome.
The treatment of choice for SAA is human leukocyte antigen (HLA)–matched sibling donor hematopoietic stem cell transplantation (HSCT), which is curative. If no sibling donor is available, immunosuppressive therapy with horse antithymocyte globulin (hATG) and cyclosporine (CSA) is recommended. Eltrombopag (choice C), a thrombopoietin-receptor agonist, has previously been shown to improve response rates when added to hATG and CSA in treatment-naïve patients. 2 However, more recent results presented at the 2019 ASH Annual Meeting did not show a similar benefit when eltrombopag was added to immunosuppressive therapy in pediatric patients. 3 A watch-and-wait approach (choice A) is typically not appropriate for patients with SAA given the ongoing risk for infection.
Although immunosuppressive therapy with hATG and CSA has led to good response rates in the pediatric population, 4 there remains an ongoing risk of relapse and later clonal evolution. Matched unrelated donor (MUD) HSCT (choice B) was historically reserved for patients with SAA who had failure to respond to immunosuppressive therapy; however, as outcomes in MUD HSCTs have improved, clinical trials are currently underway to investigate whether MUD HSCTs may be offered as a first-line therapy for patients with SAA without HLA-matched sibling donors.
References:
- Davies JK, Guinan EC. An update on the management of severe idiopathic aplastic anaemia in children . Br J Haematol. 2007;136:549-564.
- Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia . N Engl J Med. 2017;376:1540-1550.
- Groarke EM, Patel BA, Diamond C, et al. Outcomes in pediatric patients with severe aplastic anemia treated with standard immunosuppression and eltrombopag . Blood. 2019;134(Suppl 1):454.
- Scheinberg P, Wu CO, Nunez O, et al. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine . J Pediatr. 2008;153:814-819.
Case study submitted by Anu Gollapudi, MD, of Seattle Children’s Hospital, Seattle, WA.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 August 2024
Causal role of immune cells in aplastic anemia: Mendelian randomization (MR) study
- Shaojie Fu 1 ,
- Yazhe Du 2 ,
- Tingting Pan 3 ,
- Fuzhe Ma 1 ,
- Hua He 4 &
- Yuying Li 2
Scientific Reports volume 14 , Article number: 18010 ( 2024 ) Cite this article
254 Accesses
Metrics details
- Medical research
Prior research has identified associations between immune cells and aplastic anaemia (AA); however, the causal relationships between them have not been conclusively established. A two-sample Mendelian randomisation analysis was conducted to investigate the causal link between 731 immune cell signatures and AA risk using publicly available genetic data. Four types of immune signatures, including relative cell, absolute cell (AC), median fluorescence intensities and morphological parameters, were considered sensitivity analyses were also performed to verify the robustness of the results and assess potential issues such as heterogeneity and horizontal pleiotropy. Following multiple test adjustments using the False Discovery Rate (FDR) method, no statistically significant impact of any immunophenotype on AA was observed. However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction ( p of IVW < 0.01), of which eight were harmful to AA: CD127- CD8br %T cell (Treg panel), CD25 on IgD + CD38dim (B cell panel), CD38 on naive-mature B cell (B cell panel), CD39 + resting Treg % CD4 Treg (Treg panel), CD39 + secreting Treg AC (Treg panel), CD8 on CD28 + CD45RA- CD8br (Treg panel), HLA DR + NK AC (TBNK panel), Naive DN (CD4 − CD8 − ) AC (Maturation stages of T cell panel); and four were protective to AA: CD86 on CD62L + myeloid DC (cDC panel), DC AC (cDC panel), DN (CD4 − CD8 − ) NKT %T cell (TBNK panel), and TD CD4 + AC (Maturation stages of T cell panel). The results of this study demonstrate a close link between immune cells and AA by genetic means, thereby improving the current understanding of the interaction between immune cells and AA risk and providing guidance for future clinical research.
Similar content being viewed by others
Diagnostic evaluation in bone marrow failure disorders: what have we learnt to help inform the transplant decision in 2024 and beyond?
Somatic mutations in lymphocytes in patients with immune-mediated aplastic anemia

Activation of distinct inflammatory pathways in subgroups of LR-MDS
Introduction.
Aplastic anaemia (AA), characterised by bone marrow failure resulting in hypocellular marrow and pancytopenia, exhibits common symptoms such as fatigue, easy bruising or bleeding, and susceptibility to infections 1 , 2 . This condition is rare but potentially life-threatening, with a rising incidence in recent years. Current data indicates 6–9 million cases of aplastic anaemia annually in East Asia, which is 2–3 times higher than in Western countries 3 , 4 . Fortunately, advances in understanding its pathophysiology and improved treatment approaches, including hematopoietic stem cell transplantation and thrombopoietin receptor agonists, have significantly increased the survival rates of AA patients 5 , 6 . Nevertheless, aplastic anaemia remains a substantial burden for patients, their families, and the healthcare system due to its often-prolonged disease course, associated high morbidity, and the uncertainty surrounding clinical outcomes 7 .
The exact cause and pathogenesis of AA have not been fully elucidated. Numerous clinical and preclinical investigations have indicated that AA patients exhibit immune dysfunction, involving abnormalities in both cellular and humoral immunity. Notably, the effectiveness of immunosuppressive therapies (ISTs) such as antithymocyte globulin (ATG) and/or cyclosporine A (CSA) suggests the involvement of one or more immune system components in the disease's pathogenesis 2 . It appears that an elevated myeloid dendritic cell/plasmacytoid dendritic cell ratio leads to an imbalance in the T helper (Th)1/Th2 ratio in favour of Th1, ultimately resulting in abnormal activation of cytotoxic T lymphocytes (CTLs) 8 . However, the specific antigens triggering T cell responses in AA are still unknown. Further, regulatory T cells (Treg), Th17 cells, natural killer (NK) cells, memory T cells, and negative hematopoietic regulatory factors also play roles in this process 9 , 10 , 11 , 12 , 13 , 14 . Nonetheless, the precise contribution of immune cells to the development of AA remains elusive, possibly due to the intricate nature of the immune system, flaws in study design, limited sample sizes, and the influence of confounding factors not fully addressed in existing research.
Based on Mendelian independent distribution law, Mendelian randomisation (MR) is an analytical method mainly used in epidemiological aetiology inference. Recent advances in large-scale genome-wide association studies (GWAS) and MR approaches have facilitated the assessment of causal relationships between immune traits and diseases 15 , 16 , 17 . Multiple studies have substantiated the utility of MR investigations in elucidating causal relationships in blood-related disorders 18 , 19 , 20 . MR studies are adept at mitigating confounding variables and deciphering reverse causal associations within the realm of causal inference. An extensive two-sample MR analysis was used in this study to determine the causal relationship between immune cell characteristics and AA.
Study design
A two-sample MR design was established to explore the causal relationships between 731 immune cell signatures (7 groups) and AA. To ensure the reliability of the finding, each MR analysis had to fulfil the following three key assumptions 21 : (1) the instrumental variable was directly related to exposure; (2) the instrumental variable was are not related to any confounders affecting both exposure and outcome; (3) the instrumental variable had an effect on the outcome only through its effect on the exposure, without involvement in any other causal pathways. The studies included in this analysis was approved by the review boards of relevant institutional, and participants provided informed consent.
Immunity-wide Genome-wide association study (GWAS) data sources
GWAS summary statistics for each immunophenotype are available from the GWAS Catalog, accession number from GCST90001391 to GCST90002121 17 . A total of 731 immunophenotypes were covered, including of median fluorescence intensities (MFI) reflecting surface antigen levels (n = 389), absolute cell (AC) counts (n = 118), relative cell (RC) counts (n = 192) and morphological parameters (MP) (n = 32). It is important to note that the MFI, AC, and RC features encompassed various immune cell types, including B cells, CTLs, mature stages of T cells, monocytes, myeloid cells, and TBNK (T cells, B cells, natural killer cells). The MP feature, on the other hand, included panels related to CTL and TBNK cell types. The original GWAS on immune signature was performed using data from 3757 European individuals without overlapping cohorts. Approximately 22 million SNPs were genotyped using high-density arrays, and imputation was performed using a Sardinian sequence-based reference panel. Associations were measured while taking into account covariates, including age and gender 22 .
GWAS data sources for AA
GWAS summary statistics for AA were got from FinnGen and UK Biobank. A total of 473, 500 European individuals (Ncase = 4128, Ncontrol = 469,372) were included in the GWAS analysis of AA, and approximately 25 million variants were analysed after quality control and imputation 23 . Based on the source information of the participants, there is no sample overlap between the immunophenotypes and AA GWAS datasets.
Selection of instrumental variables (IVs)
The significance level of IVs for each immunophenotype was set at 1 × 10 −5 based on a recent Mendelian randomisation study on immune traits 15 . The 1000 Genomes Project linkage disequilibrium structure (r 2 < 0.1 with any other associated SNP within 10 Mb) was performed among the initially selected SNPs to ensure that the selected IVs were able to independently predict exposure. In addition, the proportion of phenotypic variation explained (PVE) and F statistic were calculated for each IV, so as to assess the strength of IV and avoid weak instrumental bias. SNPs with an F-statistic below 10 were determined to be weak instruments and subsequently ruled out from the IVs 24 . F-statistic was estimated using the formula: F = R 2 (N 2 )/(1 − R 2 ), where R 2 was the proportion of phenotypic variation explained by the SNP and N was the sample size of the GWAS of SNPs with the trait. The R 2 values were estimated using the formula: R 2 = 2 × EAF × (1 − EAF) × β 2 , where EAF was the effect allele frequency (EAF) of the SNP and β was the estimated effect of SNP on trait 25 . We also conducted a search using the PhenoScanner database to clarify whether the selected SNPs were associated with AA potential confounders (e.g., benzene exposure, ionizing radiation, organic solvents, viral infections, etc.) 20 .
Statistical analysis
All analyses were conducted using R software (version 4.3.1, https://cran.r-project.org/src/base/R-4/R-4.3.1.tar.gz ). To assess the causal link between 731 immunophenotypes and AA, statistical methods were used such as Inverse Variance Weighting (IVW), Weighted Median (WM), and Mendelian Randomisation–Egger (MR-Egger) from the 'Mendelian Randomisation' package (version 0.4.3) 26 . Heterogeneity among instrumental variables was checked using corresponding p values and Cochran's Q statistic, visualising it with a random effect model 27 . To account for potential horizontal pleiotropy, MR-Egger was applied. The MR-PRESSO method was used to identify and exclude possible pleiotropic outliers 28 . Scatter and funnel plots confirmed the robustness of the results and the absence of heterogeneity 29 . In addition, the statistical power for the MR results was calculated to clarify the probability of committing a type II statistical error for a negative result 30 .
Ethics approval and consent to participate
The study was approved by the Sardinian Regional Ethics Committee (protocol no. 2171/CE). All participants provided written, informed consent. Informed consent was obtained from all participants and/or their LAR. This study was conducted in accordance to relevant guidelines and regulations.
Development of the IVs used to genetically predict each immunophenotype
A total of 3–724 independent, non-palindromic and significant SNPs were selected as the IVs for 731 immunophenotypes. These IVs accounted for variance ranging from 0.005 to 5.199% in their respective immunophenotypes. Notably, all the genetic instruments had F statistics exceeding 10, signifying their robust strength (See Supplementary Table 1 ). In addition, as shown in Supplementary Table 2 , after searching in the PhenoScanner database, no selected SNPs were found to be associated with the AA potential confounders (e.g., benzene exposure, ionizing radiation, organic solvents, viral infections, etc.).
Exploration of the causal effect of immunophenotypes on AA
To explore the causal effects of immunophenotypes on AA, we performed a two-sample MR analysis and used the IVW method as the primary analysis. Following multiple test adjustments using the False Discovery Rate (FDR) method, no statistically significant effect of any immunophenotype on AA was observed (Supplementary Table 2 ). However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction ( p of IVW < 0.01), of which eight were harmful to AA: CD127- CD8br %T cell (Treg panel), CD25 on IgD + CD38dim (B cell panel), CD38 on naive-mature B cell (B cell panel), CD39 + resting Treg % CD4 Treg (Treg panel), CD39 + secreting Treg AC (Treg panel), CD8 on CD28 + CD45RA- CD8br (Treg panel), HLA DR + NK AC (TBNK panel), Naive DN (CD4 − CD8 − ) AC (Maturation stages of T cell panel); and four were protective to AA: CD86 on CD62L + myeloid DC (cDC panel), DC AC (cDC panel), DN (CD4 − CD8 − ) NKT %T cell (TBNK panel), and TD CD4 + AC (Maturation stages of T cell panel).
Specifically, by using the IVW method, the odds ratio (OR) of CD127- CD8br %T cell on AA risk was estimated to be 1.135 (95% CI 1.032–1.247, P = 0.009), the OR of CD25 on IgD + CD38dim on AA risk was estimated to be 1.053 (95% CI 1.013–1.095, P = 0.009), the OR of CD38 on naive-mature B cell on AA risk was estimated to be 1.073 (95% CI 1.019–1.130, P = 0.007), the OR of CD39 + resting Treg % CD4 Treg on AA risk was estimated to be 1.034 (95% CI 1.010–1.059, P = 0.005), the OR of CD39 + secreting Treg AC on AA risk was estimated to be 1.050 (95% CI 1.013–1.089, P = 0.007), the OR of CD8 on CD28 + CD45RA- CD8br on AA risk was estimated to be 1.127 (95% CI 1.044–1.215, P = 0.002), the OR of HLA DR + NK AC on AA risk was estimated to be 1.110 (95% CI 1.031–1.195, P = 0.005), the OR of Naive DN (CD4 − CD8 − ) AC on AA risk was estimated to be 1.116 (95% CI 1.032–1.207, P = 0.006), the OR of CD86 on CD62L + myeloid DC on AA risk was estimated to be 0.930 (95% CI 0.882–0.981, P = 0.008), the OR of DC AC on AA risk was estimated to be 0.897 (95% CI 0.838–0.960, P = 0.002), the OR of DN (CD4 − CD8 − ) NKT %T cell on AA risk was estimated to be 0.919 (95% CI 0.864–0.978, P = 0.007), and the OR of TD CD4 + AC on AA risk was estimated to be 0.890 (95% CI = 0.817–0.968, P = 0.007). Both the WM and MR-Egger methods, employed as complementary tests, yielded results consistent with the IVW analysis (Fig. 1 ), which reinforced the confidence in the findings. In the investigation of causality, the p value of Cochran's Q exceeded 0.05, indicating the absence of heterogeneity in the results. Additionally, MR-Egger did not detect any evidence of pleiotropy, as the p value for its intercept was greater than 0.05. Neither MR-PRESSO nor leave-one-out plots identified any outliers (Supplementary Fig. 1 ).

The significant positive MR results of 731 immunophenotypes on AA without FDR correction ( p < 0.01).
Additionally, the MR results for all immunophenotypes are shown in Supplementary Table 3 , the scatter plots, forest plots and funnel plots are presented in Supplementary Figs. 2 , 3 and 4 , respectively.
Notably, even taking the immunophenotypes related to Treg cells to primary analyses and the remaining immunophenotypes to secondary analyses, it was found that positive results were still not detected after FDR correction, as shown in Supplementary Tables 4 and 5 , respectively.
To the present knowledge, our study is the first MR study to explore the causal relationship between multiple immunophenotypes and AA. The results of this study demonstrated that no statistically significant effect of any immunophenotype on AA was found following multiple test adjustment according to the FDR method. However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction ( p of IVW < 0.01).
Dendritic cells (DCs) play a crucial role in the immune system by processing antigens for presentation to T cells and regulating their differentiation and function 31 . Previous research suggested that the activation of DCs induced by various factors can lead to abnormal activation of downstream T cells 32 , resulting in a pathological immune response, imbalance, apoptosis of bone marrow hematopoietic cells, and subsequent hematopoietic dysfunction and pancytopenia in AA 33 . It has also been observed that the number of myeloid dendritic cells (MDCs) and the expression of costimulatory molecules on DCs, including CD40, CD80, and CD86, increased in patients with severe AA from Asia 9 , 34 . MDCs exhibited strong phagocytic activity in patients with severe AA, leading to an increase in the number of CTLs 35 . Inconsistent with previous findings, our study based on European databases demonstrated that the decreased levels of DC AC and CD86 on CD62L + myeloid DC appeard to be significantly associated with the risk of AA. The inconsistency of the above results may be influenced by ethnicity, disease severity, sample size and other factors, so further research is necessary.
In the present study, associations were observed between an increased risk of AA and specific T cell-related factors. These factors included Naive DN (CD4 − CD8 − ) AC of maturation stages of T cell and four types of Treg cells ( CD127- CD8br %T cell , CD39 + resting Treg % CD4 Treg, CD39 + secreting Treg AC , CD8 on CD28 + CD45RA- CD8br ). Numerous studies have emphasised the critical role of T cells in AA, with findings indicating the importance of T cells in the disease 8 . AA patients often exhibit an elevated number of CTLs and a shift in the CD4 + to CD8 + T cell ratio. These activated CTLs tend to produce pro-inflammatory cytokines such as INF-γ and TNF-α, leading to apoptosis through the Fas/FasL pathway. This apoptosis can inhibit bone marrow's hematopoietic functions and result in hematopoietic cell destruction 2 , 7 , 36 . Furthermore, abnormalities in the number and/or function of CD4 + cells, including interferon (IFN)-γ-producing CD4 + T cells (Th1 cells), interleukin (IL)-4-producing CD4 + T cells (Th2 cells), interleukin-17 (IL-17)-producing CD4 + T cells (Th17 cells) and Tregs, have been reported in patients with AA, suggesting their potential roles in the disease's pathogenesis 8 , 37 , 38 . It has been reported that significant increases in the number of Th1 cells and the Th1/Th2 cell ratio have been observed in AA, leading to elevated production of IFN-γ, a potent stimulator of CD8 + T cells 37 , 39 . The number of Th17 cells is also increased, which induces the terminal differentiation of CTLs into effector memory CD8 + T cells via IL-17 and IL-22 stimulation, and reduces the number and function of Treg cells in AA 38 , 40 , 41 . Our study demonstrated Naive DN (CD4 − CD8 − ) AC of maturation stages of T cell was related to the risk of AA. However, due to the lack of relevant data on several subsets of CD4 + T cell in the 731 immune cell signatures, we were unable to separately analyse the causal link between Th1, Th2, and Th17 cells and risk of AA. Additionally, close correlation has been observed between the severity of AA and significant decreases in the number and functions of Treg cells, which improved with successful IST 37 , 42 . However, the presenting study demonstrated that CD39 + Treg cells ( CD39 + resting Treg % CD4 Treg and CD39 + secreting Treg AC) , which were highly active and suppressive, increased the risk of AA 43 . The role of T cells in the onset and progression of AA remains elusive. AA is a highly heterogeneous disease, and the triggering events and underlying pathogenesis may vary among patients. At the same time, immune signatures differ between early-stage or non-severe AA and late-stage or severe AA, as well as among different treatment approaches. Whether the changes in T cell profiles are the cause or the consequence of AA remains a subject of inquiry. Therefore, further research is necessary to elucidate the intricate relationship between T cells and the risk of AA 40 , 44 .
Two types of B cells (that is, CD25 on IgD + CD38dim and CD38 on naive-mature B cell ) were found to be associated with increased risk of AA. Unlike T cells, the role of B cells in AA remains unclear. Recent reports have suggested that the numbers of circulating B regulatory cells decreased at the time of diagnosis and subsequently recoverd following IST 26 . Additionally, patients with acquired AA have been found to exhibit various autoantibodies in their serum, including anti-moesin, diazepam-binding inhibitor-related protein 1 (DRS1), and kinectin antibodies, which are associated with proteins found in hematopoietic cells 40 .
In the present study, HLA DR + NK AC was found to be associated with an increased risk of AA, and the percent of DN (CD4 − CD8 − ) NKT %T cell was associated with a reduced risk of AA. Natural killer (NK) cells are innate immune system lymphocytes with effector functions. The role of NK cells in AA has shown conflicting results. In severe AA patients, both the numbers and functions of NK cells in peripheral blood have been observed to be significantly reduced, with subsequent recovery following successful IST 45 . However, a separate study focused on paediatric-acquired AA did not report a correlation between NK cell frequency and disease severity or treatment response 46 . Additionally, in non-severe AA patients, there was an increase in the percentage of CD56bright NK cells and heightened expression of the activating receptor NKG2D, while the expression of the inhibitory receptor CD158a was low. These findings suggested that the increased and activated CD56bright NK cells might have a protective role in the development of non-severe aplastic anaemia 47 .
In the present study, a two-sample MR analysis was employed based on data from large GWAS cohorts, involving approximately 48,000 individuals, thereby ensuring high statistical power. The findings were drawn from genetic instrumental variables, and causal inference was performed by various MR analysis methods. The results were robust and not influenced by horizontal pleiotropy and other potential confounders. However, several limitations need to be acknowledged. Firstly, despite conducting multiple sensitivity analyses, there remains a partial limitation in assessing horizontal pleiotropy comprehensively. Secondly, the lack of detailed information about individuals prevents further stratification within the population. Thirdly, the generalisability of the findings is limited as the study utilised European databases exclusively, making it challenging to extend the conclusions to other ethnic groups. Fourthly, the potential risk factors for AA highlighted in this study were all significant correlations exhibited without FDR correction, and thus the false positives need to be taken into account and validated by more in-depth studies in the future. Lastly, a more lenient threshold was employed to assess the results, which might increase the likelihood of false positives but also provide a more comprehensive picture of the close association between immunophenotypes and AA.
Conclusions
In conclusion, causal associations between various immunophenotypes and AA were established using a comprehensive MR analysis. Given the intricate nature of AA's pathogenesis and the evident clinical heterogeneity of immune cell types implicated in AA, the present research sheds light on the complex interplay between the immune system and AA. Moreover, the present findings mitigate the influence of confounding factors, reverse causality, and other potential biases. The present study not only offers new insights into the biological mechanisms of AA but also suggests directions for the development of novel therapeutic interventions.
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article. Further inquiries can be directed to the corresponding authors.
DeZern, A. E. & Churpek, J. E. Approach to the diagnosis of aplastic anemia. Blood Adv. 5 , 2660–2671. https://doi.org/10.1182/bloodadvances.2021004345 (2021).
Article PubMed PubMed Central Google Scholar
Young, N. S. Aplastic anemia. N. Engl. J. Med. 379 , 1643–1656. https://doi.org/10.1056/NEJMra1413485 (2018).
Article CAS PubMed PubMed Central Google Scholar
Vaht, K. et al. Incidence and outcome of acquired aplastic anemia: Real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica 102 , 1683–1690. https://doi.org/10.3324/haematol.2017.169862 (2017).
Norasetthada, L. et al. Adult aplastic anemia in Thailand: Incidence and treatment outcome from a prospective nationwide population-based study. Ann. Hematol. 100 , 2443–2452. https://doi.org/10.1007/s00277-021-04566-0 (2021).
Scheinberg, P. Acquired severe aplastic anaemia: How medical therapy evolved in the 20th and 21st centuries. Br. J. Haematol. 194 , 954–969. https://doi.org/10.1111/bjh.17403 (2021).
Article CAS PubMed Google Scholar
Drexler, B. & Passweg, J. Current evidence and the emerging role of eltrombopag in severe aplastic anemia. Ther. Adv. Hematol. 12 , 2040620721998126. https://doi.org/10.1177/2040620721998126 (2021).
Furlong, E. & Carter, T. Aplastic anaemia: Current concepts in diagnosis and management. J. Paediatr. Child Health 56 , 1023–1028. https://doi.org/10.1111/jpc.14996 (2020).
Article PubMed Google Scholar
Liu, C., Sun, Y. & Shao, Z. Current concepts of the pathogenesis of aplastic anemia. Curr. Pharm. Des. 25 , 236–241. https://doi.org/10.2174/1381612825666190313113601 (2019).
Liu, C. et al. Differential expression of the proteome of myeloid dendritic cells in severe aplastic anemia. Cell Immunol. 285 , 141–148. https://doi.org/10.1016/j.cellimm.2013.09.007 (2013).
Article ADS CAS PubMed Google Scholar
Zheng, M. et al. Abnormal immunomodulatory ability on memory T cells in humans with severe aplastic anemia. Int. J. Clin. Exp. Pathol. 8 , 3659–3669 (2015).
PubMed PubMed Central Google Scholar
Hosokawa, K. et al. Memory stem T cells in autoimmune disease: High frequency of circulating CD8 + memory stem cells in acquired aplastic anemia. J. Immunol. 196 , 1568–1578. https://doi.org/10.4049/jimmunol.1501739 (2016).
Zhang, J. et al. Single-cell analysis highlights a population of Th17-polarized CD4 + naive T cells showing IL6/JAK3/STAT3 activation in pediatric severe aplastic anemia. J. Autoimmun. 136 , 103026. https://doi.org/10.1016/j.jaut.2023.103026 (2023).
Lu, T. et al. Decreased circulating Th22 and Th17 cells in patients with aplastic anemia. Clin. Chim. Acta 450 , 90–96. https://doi.org/10.1016/j.cca.2015.07.031 (2015).
Lim, S. P. et al. Treg sensitivity to FasL and relative IL-2 deprivation drive idiopathic aplastic anemia immune dysfunction. Blood 136 , 885–897. https://doi.org/10.1182/blood.2019001347 (2020).
Wang, C. et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry 23 , 590. https://doi.org/10.1186/s12888-023-05081-4 (2023).
Gu, J. et al. Assessing the causal relationship between immune traits and systemic lupus erythematosus by bi-directional Mendelian randomization analysis. Mol. Genet. Genomics MGG 298 , 1493–1503. https://doi.org/10.1007/s00438-023-02071-9 (2023).
Orrù, V. et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 52 , 1036–1045. https://doi.org/10.1038/s41588-020-0684-4 (2020).
Li, H. et al. Mendelian randomization analysis reveals causality of inflammatory bowel disease on risks of Henoch-Schönlein purpura and immune thrombocytopenia. Dig. Liver Dis. 56 , 92–97. https://doi.org/10.1016/j.dld.2023.08.044 (2024).
Xu, P., Han, S., Hou, M., Zhao, Y. & Xu, M. The serum lipid profiles in immune thrombocytopenia: Mendelian randomization analysis and a retrospective study. Thromb. J. 21 , 107. https://doi.org/10.1186/s12959-023-00551-x (2023).
Kjaergaard, A. D. et al. Thyroid function, pernicious anemia and erythropoiesis: A two-sample Mendelian randomization study. Hum. Mol. Genet. 31 , 2548–2559. https://doi.org/10.1093/hmg/ddac052 (2022).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: Substantive and nomenclatural issues. Eur. J. Epidemiol. 35 , 99–111. https://doi.org/10.1007/s10654-020-00622-7 (2020).
Sidore, C. et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat. Genet. 47 , 1272–1281. https://doi.org/10.1038/ng.3368 (2015).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53 , 1415–1424. https://doi.org/10.1038/s41588-021-00931-x (2021).
Fu, S. et al. Effects of selenium on chronic kidney disease: A Mendelian randomization study. Nutrients https://doi.org/10.3390/nu14214458 (2022).
Wang, Q., Shi, Q., Lu, J., Wang, Z. & Hou, J. Causal relationships between inflammatory factors and multiple myeloma: A bidirectional Mendelian randomization study. Int. J. Cancer 151 , 1750–1759. https://doi.org/10.1002/ijc.34214 (2022).
Yavorska, O. O. & Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46 , 1734–1739. https://doi.org/10.1093/ije/dyx034 (2017).
Yan, D. et al. A Mendelian randomization study revealed a causal link between napping and deep vein thrombosis (DVT). Sleep Breath. https://doi.org/10.1007/s11325-023-02940-y (2023).
Lyu, B., Ma, J., Bai, Y. & Feng, Z. Casual effects of gut microbiota on risk of infections: A two-sample Mendelian randomization study. Front. Microbiol. 14 , 1284723. https://doi.org/10.3389/fmicb.2023.1284723 (2023).
Richardson, T. G., Leyden, G. M. & Davey Smith, G. Time-varying and tissue-dependent effects of adiposity on leptin levels: A Mendelian randomization study. eLife https://doi.org/10.7554/eLife.84646 (2023).
Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43 , 922–929. https://doi.org/10.1093/ije/dyu005 (2014).
Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 1 , 145–149. https://doi.org/10.1158/2326-6066.CIR-13-0102 (2013).
Ochyl, L. J. & Moon, J. J. Dendritic cell membrane vesicles for activation and maintenance of antigen-specific T cells. Adv. Healthc. Mater. 8 , e1801091. https://doi.org/10.1002/adhm.201801091 (2019).
Gao, M., Zhang, D. & Xu, R. Advances in understanding the role of dendritic cells in aplastic anaemia. Scand. J. Immunol. 97 , e13265. https://doi.org/10.1111/sji.13265 (2023).
Zonghong, S. et al. Circulating myeloid dendritic cells are increased in individuals with severe aplastic anemia. Int. J. Hematol. 93 , 156–162. https://doi.org/10.1007/s12185-010-0761-z (2011).
Sun, Y. et al. Myeloid dendritic cells in severe aplastic anemia patients exhibit stronger phagocytosis. J. Clin. Lab. Anal. 35 , e24063. https://doi.org/10.1002/jcla.24063 (2021).
Liu, C. Y. et al. Fas/FasL in the immune pathogenesis of severe aplastic anemia. Genet. Mol. Res. 13 , 4083–4088. https://doi.org/10.4238/2014.May.30.3 (2014).
Kordasti, S. et al. Functional characterization of CD4 + T cells in aplastic anemia. Blood 119 , 2033–2043. https://doi.org/10.1182/blood-2011-08-368308 (2012).
de Latour, R. P. et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood 116 , 4175–4184. https://doi.org/10.1182/blood-2010-01-266098 (2010).
Giannakoulas, N. C. et al. Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. Br. J. Haematol. 124 , 97–105. https://doi.org/10.1046/j.1365-2141.2003.04729.x (2004).
Patel, B. A., Giudice, V. & Young, N. S. Immunologic effects on the haematopoietic stem cell in marrow failure. Best Pract. Res. Clin. Haematol. 34 , 101276. https://doi.org/10.1016/j.beha.2021.101276 (2021).
Giudice, V., Cardamone, C., Triggiani, M. & Selleri, C. Bone marrow failure syndromes, overlapping diseases with a common cytokine signature. Int. J. Mol. Sci. 22 , 200. https://doi.org/10.3390/ijms22020705 (2021).
Article CAS Google Scholar
Yan, L. et al. Abnormal quantity and function of regulatory T cells in peripheral blood of patients with severe aplastic anemia. Cell Immunol. 296 , 95–105. https://doi.org/10.1016/j.cellimm.2015.04.001 (2015).
Álvarez-Sánchez, N. et al. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci. Rep. 9 , 2302. https://doi.org/10.1038/s41598-019-38897-w (2019).
Giudice, V. & Selleri, C. Aplastic anemia: Pathophysiology. Semin. Hematol. 59 , 13–20. https://doi.org/10.1053/j.seminhematol.2021.12.002 (2022).
Liu, C. et al. Abnormalities of quantities and functions of natural killer cells in severe aplastic anemia. Immunol. Invest. 43 , 491–503. https://doi.org/10.3109/08820139.2014.888448 (2014).
Sutton, K. S., Shereck, E. B., Nemecek, E. R. & Kurre, P. Immune markers of disease severity and treatment response in pediatric acquired aplastic anemia. Pediatr. Blood Cancer 60 , 455–460. https://doi.org/10.1002/pbc.24247 (2013).
Li, Y. et al. Abnormalities of quantities and functions of CD56bright natural killer cells in non-severe aplastic Anemia. Hematology 24 , 405–412. https://doi.org/10.1080/16078454.2019.1590963 (2019).
Download references
Acknowledgements
The authors thank the contributors to the ieu open gwas project ( https://gwas.mrcieu.ac.uk/ ) for sharing data.
This study was supported by grants from the Health Special Project of Jilin Province (No. JLSWSRCZX2020-0065) and Natural Science Foundation of Jilin Province (No. 20210101259JC).
Author information
Authors and affiliations.
Department of Nephrology, The First Hospital of Jilin University, Changchun, China
Shaojie Fu & Fuzhe Ma
Department of Hematology, The First Hospital of Jilin University, Changchun, China
Yazhe Du & Yuying Li
Teaching Department, The First Hospital of Jilin University, Changchun, China
Tingting Pan
Department of Oncology, The First Hospital of Jilin University, Changchun, China
You can also search for this author in PubMed Google Scholar
Contributions
Y.L. and S.F. designed the experiments. S.F. and Y.D. carried out the experiments. F.M. and T.P. analyzed the data. S.F. and T.P. wrote the manuscript. H.H. contributed to statistical analysis. Y.L. supervised the study and revised the paper. All authors have read and agreed to the published version of the manuscript. All authors agree to publish.
Corresponding author
Correspondence to Yuying Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary Information
Supplementary information 1., supplementary information 2., supplementary information 3., supplementary information 4., supplementary information 5., supplementary information 6., supplementary information 7., supplementary information 8., supplementary information 9., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fu, S., Du, Y., Pan, T. et al. Causal role of immune cells in aplastic anemia: Mendelian randomization (MR) study. Sci Rep 14 , 18010 (2024). https://doi.org/10.1038/s41598-024-69104-0
Download citation
Received : 10 November 2023
Accepted : 31 July 2024
Published : 03 August 2024
DOI : https://doi.org/10.1038/s41598-024-69104-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aplastic anaemia
- Causal inference
- Mendelian randomisation study
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A case-control study of aplastic anemia
Affiliation.
- 1 Department of Epidemiology, Johns Hopkins School of Hygiene and Public Health, Baltimore, MD.
- PMID: 2915573
- DOI: 10.1016/0145-2126(89)90025-8
A case-control interview study of aplastic anemia was conducted to evaluate suspected risk factors. Cases (N = 59) newly diagnosed during 1975-82 at 25 Baltimore area hospitals were compared with 59 individually matched (on age, sex and race) controls selected by random digit dialing. The average educational level was less for cases than controls. The major job-related findings were a significant excess for occupational exposure to paint (OR = 6.1; 95% C.I. = 1.2-29.7), further substantiated by a positive dose-response relationship, although painters were not at excess risk. An increased risk of occupational exposure to viruses (OR = 9.0; 95% C.I. = 0.8-105.6) was noted. Additional evidence implicating viral factors included a significant association with prior history of hepatitis (OR = 9.0; 95% C.I. = 1.0, 84.2) and an elevated risk for pre-diagnostic receipt of blood transfusions (OR = 7.1; 95% C.I. = 0.7-68.4). Risks were not increased for other occupational, residential, personal, or medical treatment exposures or for other viral infections, medical conditions, smoking or alcohol consumption prior to diagnosis. Because of the small number of subjects studied and the multiple comparisons examined, these findings should be interpreted cautiously and confirmation should be undertaken in larger, population-based studies.
PubMed Disclaimer
Similar articles
- The role of occupational and environmental exposures in the aetiology of acquired severe aplastic anaemia: a case control investigation. Muir KR, Chilvers CE, Harriss C, Coulson L, Grainge M, Darbyshire P, Geary C, Hows J, Marsh J, Rutherford T, Taylor M, Gordon-Smith EC. Muir KR, et al. Br J Haematol. 2003 Dec;123(5):906-14. doi: 10.1046/j.1365-2141.2003.04718.x. Br J Haematol. 2003. PMID: 14632783
- An apparent cluster of aplastic anemia in a small population of teenagers. Linet MS, Tielsch JM, Markowitz JA, Sensenbrenner LL, McCaffrey LD, Warm SG, Vanderslice SF, Morgan WF, Bearden JD 3rd, Szklo M. Linet MS, et al. Arch Intern Med. 1985 Apr;145(4):635-40. Arch Intern Med. 1985. PMID: 3985725
- Lack of known hepatitis virus in hepatitis-associated aplastic anemia and outcome after bone marrow transplantation. Safadi R, Or R, Ilan Y, Naparstek E, Nagler A, Klein A, Ketzinel-Gilaad M, Ergunay K, Danon D, Shouval D, Galun E. Safadi R, et al. Bone Marrow Transplant. 2001 Jan;27(2):183-90. doi: 10.1038/sj.bmt.1702749. Bone Marrow Transplant. 2001. PMID: 11281388
- [The etiology of aplastic anemia]. Abdulkadyrov KM, Bessmel'tsev SS, Shilova ER. Abdulkadyrov KM, et al. Lik Sprava. 1995 Sep-Dec;(9-12):16-21. Lik Sprava. 1995. PMID: 8983764 Review. Russian. No abstract available.
- Aplastic anemia after transplantation for non-A, non-B, non-C fulminant hepatic failure: case report and review of the literature. Itterbeek P, Vandenberghe P, Nevens F, Fevery J, Aerts R, Yap SH, Demuynck H, Fourneau I, Koshiba T, Emonds MP, Roskams T, Boogaerts M, Pirenne J. Itterbeek P, et al. Transpl Int. 2002 Mar;15(2-3):117-23. doi: 10.1007/s00147-002-0383-3. Epub 2002 Mar 5. Transpl Int. 2002. PMID: 11935168 Review.
Publication types
- Search in MeSH
Related information
Grants and funding.
- R01 CA 24757/CA/NCI NIH HHS/United States
LinkOut - more resources
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Subscribe or Renew
Create an E-mail Alert for This Article
Somatic mutations and clonal hematopoiesis in aplastic anemia, permissions, information & authors, metrics & citations, view options, conclusions, study design, dna sequencing and single-nucleotide polymorphism array, statistical analysis, targeted sequencing and snp array karyotyping.

Clinical Correlations

Chronology of Clonal Architecture in Aplastic Anemia

Supplementary Material
Information, published in.
- Hematology/Oncology General
Affiliations
Export citation.
Select the format you want to export the citation of this publication.
- Agnieszka Piekarska,
- Katarzyna Pawelec,
- Anna Szmigielska-Kapłon,
- Marek Ussowicz,
- Qianshuang Geng,
- Guoxiang Zhang,
- Mingxia Wei,
- Daria V. Babushok,
- Amy E. DeZern,
- Carlos M. de Castro,
- Zora R. Rogers,
- David Beenhouwer,
- Michael S. Broder,
- Suzanne R. Fanning,
- Sarah N. Gibbs,
- Rabi Hanna,
- Jaroslaw P. Maciejewski,
- Bart L. Scott,
- Srinivas K. Tantravahi,
- Marcin W. Wlodarski,
- Irina Yermilov,
- Bhumika J. Patel,
- Margarete A. Fabre,
- George S. Vassiliou,
- Rashmi Kanagal-Shamanna,
- David B. Beck,
- Katherine R. Calvo,
- Joshua D. Strauss,
- Derek W. Brown,
- Weiyin Zhou,
- Casey Dagnall,
- Jian‐Min Yuan,
- Sharon A. Savage,
- Youjin Wang,
- Maryam Rafati,
- Stephen R. Spellman,
- Shahinaz M. Gadalla,
- Tomoya Maeda,
- Akira Matsuda,
- Junya Kanda,
- Hiroshi Kawabata,
- Takayuki Ishikawa,
- Kaoru Tohyama,
- Akira Kitanaka,
- Kayano Araseki,
- Kei Shimbo,
- Tomoko Hata,
- Takahiro Suzuki,
- Hidekazu Kayano,
- Kensuke Usuki,
- Maki Shindo‐Ueda,
- Nobuyoshi Arima,
- Masaharu Nohgawa,
- Akiko Ohta,
- Shigeru Chiba,
- Yasushi Miyazaki,
- Shinji Nakao,
- Keiya Ozawa,
- Shunya Arai,
- Mineo Kurokawa,
- Akifumi Takaori‐Kondo,
- Kinuko Mitani,
- Austin Kulasekararaj,
- Jamie Cavenagh,
- Inderjeet Dokal,
- Theodora Foukaneli,
- Shreyans Gandhi,
- Mamta Garg,
- Morag Griffin,
- Peter Hillmen,
- Robin Ireland,
- Sally Killick,
- Sahar Mansour,
- Ghulam Mufti,
- Victoria Potter,
- John Snowden,
- Simon Stanworth,
- Roslin Zuha,
- Judith Marsh,
- Xie bing Bao,
- Ningzheng Dong,
- Suning Chen,
- Peng-Yu Li,
- Mei-Hong Fu,
- Yong-Mei Zhu,
- Jian-Feng Li,
- Wei-Ping Yang,
- Wang-Wei Cai,
- Rui-Bao Ren,
View options
Content link.
Copying failed.
PREVIOUS ARTICLE
Next article, more from vol. 373 no. 1.
- Original Article
- Jul 02, 2015
Afamelanotide for Erythropoietic Protoporphyria
Combined nivolumab and ipilimumab or monotherapy in untreated melanoma, a randomized, controlled trial of 3.0 mg of liraglutide in weight management.
JANSSEN GLOBAL CHANGE LOCATION >
Groundbreaking nipocalimab study of pregnant individuals at high risk for early onset severe hemolytic disease of the fetus and newborn published in The New England Journal of Medicine
Nipocalimab delayed or prevented severe fetal anemia and 54 percent of study participants in the Phase 2 UNITY study achieved a live birth at or after 32 weeks without the need for intrauterine transfusion (IUT)
The AZALEA Phase 3 clinical study is currently enrolling patients: Nipocalimab is the only therapy in clinical development for use in pregnancies at risk for severe hemolytic disease of the fetus and newborn (HDFN)
SPRING HOUSE, Pa., (August 7, 2024) – Johnson & Johnson today announced the results from the Phase 2 open-label UNITY study of nipocalimab for the treatment of alloimmunized a pregnant individuals at risk of early onset severe (EOS) HDFN have been published in The New England Journal of Medicine (NEJM). The UNITY study met its primary endpoint with 54 percent of individuals receiving nipocalimab achieving a live birth at or after 32 weeks gestational age (GA) without the need for IUT. 1 Nipocalimab is currently the only therapy reported to be in clinical development for HDFN, a serious and rare condition that occurs when the blood types of a pregnant individual and the developing fetus are incompatible, potentially causing life-threatening anemia in the fetus or infant. 2 These results showed that nipocalimab delayed or prevented severe fetal anemia requiring treatment prenatally and reduced the need for IUTs in pregnancies at high risk for recurrent EOS HDFN. 1
“The Phase 2 data published in the NEJM are encouraging, as the results support the potential of nipocalimab in the treatment of pregnant individuals with a history of severe HDFN, helping to establish a path forward for further development in this disease in a larger scale Phase 3 study,” said Kenneth J. Moise Jr., M.D., Professor, Department of Women’s Health and Co-Director, Comprehensive Fetal Care Center at Dell Medical School of the University of Texas at Austin and lead study investigator b . “For many patients, severe HDFN has a poor prognosis, and the current standard of care carries with it a high treatment burden, such as repeated IUTs and additional in-utero procedures that require access to specialty care and carry a risk to the life of the fetus. If approved, nipocalimab would be the first non-surgical treatment for pregnancies at high risk of HDFN.” 3
The multicenter, open-label, single-arm Phase 2 UNITY study assessed intravenous nipocalimab from 14-35 weeks in pregnancies at high risk for recurrent EOS HDFN. 1 The primary endpoint of the study is live birth at ≥32 weeks GA without IUT. Study results showed the primary endpoint was achieved in 54 percent (7/13) of pregnancies versus the 10 percent historical benchmark (95 percent CI, 25.1-80.8; P<0.001). 1 The NEJM manuscript includes new data that compares the outcomes of qualifying pregnancies c and on-study (UNITY) pregnancies. 1 The comparison revealed that study pregnancies had a higher proportion of live births (92 percent versus 38 percent), fewer participants requiring IUTs (85 percent versus 46 percent), a later median GA at first IUT (27 and 1/7 weeks versus 20 and 4/7 weeks) and a later median GA at delivery (36 4/7 weeks versus 23 and 6/7 weeks). 1 Additionally, among pregnant individuals who joined the study, seven had a fetus that developed hydrops in their most recent qualifying pregnancy, whereas no incidences of hydrops occurred in the study pregnancies. 1
In the UNITY study, the most frequently reported adverse events were consistent with those common in pregnancy and HDFN. 1 Serious side effects were consistent with HDFN or other pregnancy-related conditions including subchorionic hematoma and premature separation of the placenta. 1 Infections and illnesses in infants of mothers exposed to nipocalimab were consistent with those typically observed in the neonatal and infancy period. 1 No maternal or neonatal/infant deaths occurred in the study. 1 One pregnancy resulted in fetal demise related to a complication of an IUT. 1
The UNITY study demonstrated positive efficacy and safety results which supports a favorable benefit risk profile for nipocalimab. 1 Thus, the UNITY study results support further clinical development of nipocalimab for the treatment of severe HDFN. 1 The AZALEA Phase 3 pivotal study is currently enrolling pregnant individuals at risk for severe HDFN who have a history of severe HDFN in a prior pregnancy(ies) to further assess the efficacy and safety of nipocalimab. 4 In addition, Johnson & Johnson is conducting a Phase 3 study of nipocalimab in fetal and neonatal alloimmune thrombocytopenia (FNAIT), which has been considered to be the platelet counterpart of HDFN. 5 FNAIT is an alloimmune disorder of pregnancy that results when the pregnant person’s immune system attacks fetal or newborn platelets, resulting in thrombocytopenia and risk of bleeding, which can be life-threatening. 6
“We are committed to developing non-surgical options that are effective and have a proven safety profile for the treatment of alloantibody-driven maternal-fetal diseases,” said Katie Abouzahr, M.D., Vice President, Autoantibody Diseases and Maternal-Fetal Immunology Disease Area Leader, Johnson & Johnson Innovative Medicine. “The data published in the NEJM underscore the potential of nipocalimab to address the high unmet medical need in severe HDFN , a serious, life-threatening and rare condition in which no other therapies in clinical development exist.”
Editor’s Notes:
a. Alloimmunized: immune response to foreign antigens upon exposure to genetically different cells or tissues b. Dr. Kenneth Moise is a paid consultant for Janssen. He has not been compensated for any media work. c. Most recent qualifying pregnancy: previous HDFN pregnancy that made the participant eligible for the UNITY Phase 2 study
ABOUT THE UNITY STUDY
UNITY ( NCT03842189 ) is a global, multicenter, non-blinded Phase 2 clinical study designed to evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of nipocalimab for the treatment of pregnant individuals at high risk for early-onset severe (EOS)-HDFN. 7 The study enrolled RhD (D) or Kell (K) alloimmunized pregnant individuals with singleton pregnancies at high risk for EOS-HDFN due to an obstetric history of severe fetal anemia, fetal hydrops, or a stillbirth at ≤24 weeks GA. 1 The primary endpoint was live birth at or after GA of 32 weeks, without a need for an intrauterine transfusion (IUT) throughout the entire pregnancy. 1 Safety was monitored for 24 weeks post-delivery for the 13 maternal individuals enrolled, and up to 96 weeks post-birth for infants. Participants received once-weekly intravenous infusions. 1 The study met the primary endpoint, with 54 percent of pregnant participants who received nipocalimab achieving a live birth at or after 32 weeks GA, without the need for an IUT throughout their entire pregnancy. 1
Hemolytic disease of the fetus and newborn (HDFN) is a rare disease (and in its severe form, ultra rare) that arises in pregnancies with maternal-fetal incompatibility in certain red blood cell types. 8 Alloantibodies produced by the maternal immune system against fetal red blood cells cross the placenta during pregnancy and attack fetal red blood cells causing fetal anemia or persist after birth in the neonate to cause neonatal hyperbilirubinemia and anemia. 2 The symptoms of HDFN can range from mild jaundice, to neurotoxic hyperbilirubinemia in the newborn, to life-threatening fetal anemia requiring invasive intervention. 9 The potential for in utero onset at an increasingly earlier GA with increasing risk of severe outcomes may occur with each incompatible pregnancy due to pregnancy-related alloimmunization. 10 Currently no non-surgical interventions are approved for pregnancies at high risk for severe HDFN. 3 Pregnancies affected by severe HDFN may necessitate repeated intrauterine transfusions (IUTs), which are invasive, technically complex surgical procedures performed by specialists at specialized medical centers, and these procedures are associated with an increased rate of fetal mortality and premature birth. 11 , 12 , 13 The most difficult to treat cases of HDFN are early onset severe HDFN (EOS-HDFN) that develops at ≤24 weeks gestational age (GA) and results in significant fetal/neonatal morbidity and mortality. According to the American Journal of Obstetrics and Gynecology , in the U.S., it is estimated that up to 80 of every 100,000 pregnancies are affected by HDFN each year. 14
ABOUT NIPOCALIMAB
Nipocalimab is an investigational monoclonal antibody, purposefully designed to bind with high affinity to block FcRn and reduce levels of circulating immunoglobulin G (IgG) antibodies, while preserving immune function without causing broad immunosuppression. This includes autoantibodies and alloantibodies that underlie multiple conditions across three key segments in the autoantibody space including Rare Autoantibody diseases, Maternal-Fetal diseases mediated by maternal alloantibodies and Prevalent Rheumatology. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Blockade of IgG binding to FcRn in the placenta is also believed to prevent transplacental transfer of maternal alloantibodies to the fetus. 24 , 25
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) have granted several key designations to nipocalimab including:
- Fast Track designation in hemolytic disease of the fetus and newborn (HDFN) and warm autoimmune hemolytic anemia (wAIHA) in July 2019, gMG in December 2021 and fetal neonatal alloimmune thrombocytopenia (FNAIT) in March 2024
- Orphan drug status for wAIHA in December 2019, HDFN in June 2020, gMG in February 2021, chronic inflammatory demyelinating polyneuropathy CIDP in October 2021 and FNAIT in December 2023
- Breakthrough Therapy designation for HDFN by the FDA in February 2024
- Orphan medicinal product designation for HDFN by the EMA in October 2019
ABOUT JOHNSON & JOHNSON
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity.
Learn more at https://www.jnj.com/ or at www.janssen.com/johnson-johnson-innovative-medicine .
Follow us at @JanssenUS and @JNJInnovMed .
Janssen Research & Development, LLC and Janssen Biotech, Inc. are Johnson & Johnson companies.
Media contact: Bridget Kimmel Mobile: (215) 688-6033 [email protected]
Investor contact: Raychel Kruper [email protected]
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding product development and the potential benefits and treatment impact of nipocalimab. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Janssen Research & Development, LLC, Janssen Biotech, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov , www.jnj.com or on request from Johnson & Johnson. None of Janssen Research & Development, LLC, Janssen Biotech, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
Source: Johnson & Johnson
[1] Kenneth J. Moise Jr., et al. Nipocalimab in Early-onset Severe Hemolytic Disease of the Fetus & Newborn. N Engl J Med. 2024; DOI: 10.1056/NEJMoa2314466.
[2] National Library of Medicine. Hemolytic Diseases of the Newborn. StatPearls Publishing. 2023 Jan. Available at: https://www.ncbi.nlm.nih.gov/books/NBK557423/ . Last accessed: August 2024.
[3] DeMoss, P., Asfour, M. and Hersey, K. Anti-K1 (Kell) antibody expressed in maternal breastmilk: A case report of a neonate with multiple intrauterine transfusions and postnatal exposure to Kell antibody in maternal breastmilk’, Case reports in pediatrics. 2017. Doi:10.1155/2017/6927813. Last accessed: August 2024.
[4] Clinicaltrials.gov. A Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of M281 Administered to Pregnant Women at High Risk for Early Onset Severe Hemolytic Disease of the Fetus and Newborn (HDFN). Last accessed: August 2024. https://clinicaltrials.gov/ct2/show/NCT03842189 .
[5] Orphanet. Fetal and Neonatal Alloimmune Thrombocytopenia. https://www.orpha.net/en/disease/detail/853 . Last accessed: August 2024.
[6] NORD. Fetal and Neonatal Alloimmune Thrombocytopenia. Published online July 2022. https://rarediseases.org/rare-diseases/fetal-and-neonatal-alloimmune-thrombocytopenia/ . Last accessed August 2024.
[7] ClinicalTrials.gov Identifier: NCT03842189. Available at: https://clinicaltrials.gov/study/NCT03842189 . Last accessed: August 2024.
[8] Hemolytic disease of the newborn. Medline Plus. Last accessed: August 2024. https://medlineplus.gov/ency/article/001298.htm
[9] Ree IMC, Smits-Wintjens VEHJ, van der Bom JG, et al. Neonatal management and outcome in alloimmune hemolytic disease, Expert Review of Hematology, 10:7, 607-616, doi: 10.1080/17474086.2017.1331124. Last accessed: August 2024.
[10] Lobato G, Soncini CS. Relationship between obstetric history and Rh(D) alloimmunization severity. Arch Gynecol Obstet. 2008 Mar;277(3):245-8. Doi: 10.1007/s00404-007-0446-x. Last accessed: August 2024.
[11] Texas Children’s Hospital. Intrauterine Transfusion. Available at: https://women.texaschildrens.org/program/texas-childrens-fetal-center/procedures-offered/intrauterine-transfusion . Last accessed: August 2024.
[12] de Winter DP, Kaminski A, et al. Hemolytic disease of the fetus and newborn: systematic literature review of the antenatal landscape. BMC Pregnancy and Childbirth. 2023;23(12). Doi: https://doi.org/10.1186/s12884-022-05329-z . Last accessed: August 2024.
[13] Lindenburg IT, van Kamp IL, van Zwet EW, Middeldorp JM, Klumper FJ, Oepkes D. Increased perinatal loss after intrauterine transfusion for alloimmune anaemia before 20 weeks of gestation. BJOG. 2013 Jun;120(7):847-52. doi: 10.1111/1471-0528.12063.
[14] Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematology Am Soc Hematol Educ Program. (2015) 2015(1):146-151. doi: https://doi.org/10.1182/asheducation-2015.1.146 . Last accessed: August 2024.
[15] ClinicalTrials.gov Identifier: NCT04951622. Available at: https://clinicaltrials.gov/ct2/show/NCT04951622 . Last accessed: August 2024.
[16] ClinicalTrials.gov. NCT03842189. Available at: https://clinicaltrials.gov/ct2/show/NCT03842189 . Last accessed: August 2024.
[17] ClinicalTrials.gov Identifier: NCT05327114. Available at: https://www.clinicaltrials.gov/study/NCT05327114 . Last accessed: August 2024.
[18] ClinicalTrials.gov Identifier: NCT04119050. Available at: https://clinicaltrials.gov/study/NCT04119050 . Last accessed: August 2024.
[19] ClinicalTrials.gov Identifier: NCT05379634. Available at: https://clinicaltrials.gov/study/NCT05379634 . Last accessed: August 2024.
[20] ClinicalTrials.gov Identifier: NCT05912517. Available at: https://www.clinicaltrials.gov/study/NCT05912517 . Last accessed: August 2024
[21] ClinicalTrials.gov Identifier: NCT06028438. Available at: https://clinicaltrials.gov/study/NCT06028438 . Last accessed: August 2024.
[22] ClinicalTrials.gov Identifier: NCT04968912. Available at: https://clinicaltrials.gov/study/NCT04968912 . Last accessed: August 2024.
[23] ClinicalTrials.gov Identifier: NCT04882878. Available at: https://clinicaltrials.gov/study/NCT04882878 . Last accessed: August 2024.
[24] Lobato G, Soncini CS. Relationship between obstetric history and Rh(D) alloimmunization severity. Arch Gynecol Obstet. 2008 Mar;277(3):245-8. DOI: 10.1007/s00404-007-0446-x. Last accessed: June 2024.
[25] Roy S, Nanovskaya T, Patrikeeva S, et al. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. Am J Obstet Gynecol. 2019;220(5):498 e491-498 e499.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ethiop J Health Sci
- v.33(1); 2023 Jan
A Novel Case Report of Severe Aplastic Anemia with COVID Infection
Fatemeh nejatifar.
1 Department of Hematology and Oncology, Guilan University of Medical Sciences, Rasht, Iran
2 Department of infectious diseases , Guilan University of Medical Sciences, Rasht, Iran
Ali Akbar Samadani
3 Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran
Aplastic anemia is a rare disease of the hematopoietic system. Although some viral agents have been implicated, the association between COVID-19 and aplastic anemia is unclear. In this way, several cases of aplastic anemia have been reported following infection with COVID-19. Importantly, we reported a 16-year-old girl with severe aplastic anemia with no history of disease following an Omicron infection who did not respond well to treatment despite supportive treatment and immunosuppression.
Introduction
Aplastic anemia (AA) is a seldom hematological position that is indicated by the main pancytopenia because of bone marrow failure. In this way, it is confirmed that this type of special bone marrow failure syndrome is the result of the destruction of hematopoietic stem cells (HPSC) that is caused due to the dysregulated auto-immune signal. For the etiology of this disease, we can say that ionizing radiation, chemotherapy, and also viral infections are associated with this disease ( 1 ).
Although COVID has more pulmonary manifestations, involvement of various organs including the hematopoietic system has been reported. Hematologic disorders include Lymphopenia, thrombocytopenia, anemia, and other common complications. It is noteworthy that the relationship between aplastic anemia and COVID is still unknown. ( 2 , 3 ). In this case report, we explain a rare case of severe aplastic anemia with the COVID-19 virus.
Case Presentation
The patient was a 16-year-old woman with severe weakness, lethargy, and blurred vision during the sixth peak of COVID-19 (Omicron) in Iran. In this way, she had a history of fever and chills with myalgia and loss of appetite, sore throat, and dry cough in the last week. She had also, a mild fever with chills and dry cough without hemoptysis and epistaxis with no evidence of bleeding mentioned. Remarkably, it did not indicate shortness of breath, chest discomfort, or chest pain. There was also disclosed contact history of a suspect with COVID-19 family members. The second dose of the Sinopharm vaccine was injected one week before the disease. She has been receiving Symptomatic treatment at home for a week due to fever and cough, and she has been referred to the center due to the aggravation of her symptoms and severe weakness, bullerd vision and menorrhagia, and also lethargy. During the initial emergency examination, severe pancytopenia was diagnosed and the patient was admitted to the center. Conspicuously, no history of previous illness, medication, recent travel, contact with animals, or tick bites was reported. Clinical examination detected slight erythema on throat examination without exudate and mucosal lesions or Petechiae, and evidence of bleeding in the mouth and gums. The pale conjunctiva was seen without jaundice and evidence of bleeding. Normal abdominal and pelvic examination was diagnosed without organomegaly and lymphadenopathy. Retinal hemorrhage was seen in both eyes and was preferred on the right eye examination. Vital signs were included like Bp(blood pressure)80 /40 mmHg, PR(pulse rate)113 beats per minute, RR(respiratory rate)18 breaths per minute, BT(body temperature)36.5c 0 , o2sat 100 % and preliminary tests were comprised Wbc=800 10 3 /microliter, Hbg=3.9 g/dl, Plt =6000 /10 3 /microliter, Esr= 135 mm/hour, Procalcitonin 30 ng/ml. Additionally, other tests were done due to severe Neutropenia and Systolic blood pressure of 80 with Tachycardia. Meanwhile, the blood and urine cultures were sent to the laboratory for more consideration ( Table 1 ).
Important variables that were measured
| Variable | DAY1 | DAY9 | DAY14 | Normal Range |
| blood sugar(mg/dl) | 151 | |||
| Bun(mg/dl) | 10 | 7–21 | ||
| Creatinine(mg/dl) | 0.96 | 0.6–1.3 | ||
| Bilirubin(Total) (mg/dl) | 0.5 | 0.3–1.2 | ||
| Bilirubin(Direct) (mg/dl) | 0.2 | 0–0.2 | ||
| SGOT(U/L) | 13 | <41 | ||
| SGPT(U/L) | 30 | <41 | ||
| Alkaline phosphatase(U/L) | 342 | 70–290 | ||
| CPK(U/L) | 20 | >140 | ||
| LDH(U/L) | 210 | 240–480 | ||
| Amylase(U/L) | 12 | <90 | ||
| Phosphorus(mg/dl) | 4.4 | 2.7–4.5 | ||
| Na(mEq/l) | 139 | 135–145 | ||
| K(mEq/l) | 3.7 | 3.5–5.3 | ||
| MAGNESIUM(mg/dl) | 2.8 | 1.53–2.55 | ||
| Lipase(U/L) | 17 | <=60 | ||
| CRP(mg/L) | 207 | <10 | ||
| White-cell count(x10^3/UL) | 0.8 | 2.7 | 2.6 | 4–11 |
| Red-cell count (x10^3/UL) | 1.33 | 2.62 | 2.87 | 4.5–5.1 |
| Hemogelobin(g/dL) | 3.9 | 7.9 | 8.5 | 12.3–15.3 |
| Heamatocrit% | 13.3 | 25.3 | 27.3 | 34.5–44.6 |
| M.C.V(FL) | 100 | 96.6 | 95.1 | 80–100 |
| M.C.H(pg) | 29.3 | 30.2 | 29.6 | |
| M.C.H.C(gr/dl) | 29.3 | 31.2 | 31.1 | 31–37 |
| Platelet(x10^3/UL) | 6 | 53 | 27 | 150–450 |
| Neutrophil% | 9 | 7 | ||
| Lymphocyte% | 89 | 90 | ||
| Monocyte% | 2 | 2 | ||
| Anisocytosis | + | |||
| Hypochromia | + | |||
| ESR 1hr(mm/hr) | 135 | Up to 30 | ||
| VBG | 110 | 90–110 | ||
| Chloride(mEq/l) | 7.4 | |||
| PH | 39.5 | |||
| Pco2(mmHg) | 21 | |||
| Po2(mmHg) | 24.7 | |||
| Hco3(mEq/l) | 35.5 | 95–100 | ||
| O2 Sat% | _0.1 | _2 to +2 | ||
| Be(mmol/L) | 0 | |||
| BB(mmol/L) | 13.3 | 11–13.5 | ||
| PT(Sec) | 12 | |||
| Protrombin.control(Sec) | 1.2 | 1–1.3 | ||
| INR | 28 | 28–40 | ||
| PTT(Sec) | ||||
| Urinanalysis | ||||
| Urinanalysis | Yellow | |||
| Color | Semi Clear | |||
| Appearance | 1010 | |||
| Specific gravit | 7 | |||
| PH | Neg | |||
| Urine protein | Trace | |||
| Glucose | Neg | |||
| Bilirubin | Neg | |||
| Urobilinogen | Neg | |||
| Keton | 1+ | |||
| Blood | 2–3 | |||
| White-cell count(perµgl)(x10^3/UL) | 8–10 | |||
| Red-cell count(perµgl)) (x10^3/UL) | 6–8 | |||
| Epithelial cell | Neg | |||
| Nitrite | No growth | |||
| Urine culture & Sensitivity | E.coli | |||
| Blood Culture X2 | 0.3 | Up to1.5 | ||
| Reticulocyte count | 158 | 200–400 | ||
| Fibrinogen | 674 | |||
| VitaminB12(pg/ml) | ||||
In this account, broad-spectrum antibiotics including Meropenem and Ciprofloxacin were started together with Vancomycin. Relatively, CXR(chest x-ray) and abdominal and pelvic ultrasound were performed. On the X-ray of the chest, a slight increase in bilateral broncho vascular marking without consolidation was observed and the COVID-19 RT-PCR was positive. The patient was treated with Remdesivir 200 mg initial dose and continued 100 mg daily for 5 days with Dexamethasone 6 mg daily. Supportive treatment including packed cell, blood, and platelet transfusion was performed due to retinal bleeding. bone marrow aspiration and biopsy were done. In the bone marrow study, cellularity decreased by 5-10% without increasing blast and CD 34 was less than 1 percent ( Figures 1 and and2). 2 ). In flow cytometry of bone marrow, severely hypocellular marrow and normal B- and T cells were reported. Secondary causes of aplastic anemia including hepatitis B, C, HIV, CMV, Parvovirus b19, and EBV were reported negative. After completing COVID-19 treatment, treatment with cyclosporin 3 mg per kg, Romiplostim, and erythropoietin was started. The patient was discharged in good general condition and was followed up on an outpatient basis. Correspondingly, the patient was a candidate for bone marrow allogeneic transplantation and the donor did not have a proper sibling and the search for an unrelated donor began. In most COVID cases, pancytopenia is transient and mild and does not require bone marrow examination.

Immunohistochemical stains reveal decreased number of cd34 cells

Biopsy and aspiration of bone marrow show severe hypocellular marrow for age.
COVID-19, which is caused by the SARS-COVID virus, has been an epidemic since 2019 and causes a mild to fatal disease that has multisystem symptoms. Hematologic disorders are common in this disease. One of the rare cases of aplastic anemia is viral causes. Various mechanisms have been proposed for the occurrence of bone marrow failure in viral infections, including destabilizing and increasing the production of inflammatory cytokines and destruction of hematopoietic cells by the virus. In this account, some viruses have been linked to this disease, but the association between COVID and aplastic anemia is not yet known ( 4 ). In most cases, pancytopenia is transient and mild and does not require bone marrow examination. Vikram et al., reported a 29-year-old woman with a history of seizures, developing aplastic anemia after COVID, which responded after several courses of immunosuppressive therapy. Our patient did not respond well to treatment and needed a blood transfusion and platelets. Ranjima et al. reported a 4-year-old girl who developed aplastic anemia after COVID, Which did not satisfactorily respond to treatment and became a candidate for bone marrow transplantation ( 5 ). A 25-year-old woman with a history of primary glomerulonephritis, who developed aplastic anemia after COVID, did not respond well after two months of immunosuppressive therapy and became a candidate for a bone marrow transplant. Our patient, without a history of any specific disease, developed severe aplastic anemia after COVID-19, which did not respond well to supportive and immunosuppressive therapy. A 53-year-old man with a history of mantle-cell lymphoma who underwent autologous bone marrow transplantation and is currently receiving monoclonal antibody treatment was admitted with pancytopenia and severe respiratory distress. After 45 days, COVID PCR was positive in peripheral blood and bone marrow. Antiviral therapy was used to treat pancytopenia. The cause of pancytopenia was explained to be bone marrow infection with the virus. COVID-19 can cause hematologic symptoms such as thrombocytopenia and lymphopenia, anemia, but giving pancytopenia and reducing cellularity by up to 5% is rare and few cases have been reported. The pathogenesis of COVID in the development of aplastic anemia and the course and mediation of these patients is not completely clear.
Acknowledgment
The authors express their gratitude and appreciation to all persons who contributed to this manuscript. The ethical code is IR.GUMS.REC.1401.084.
- Case Report
- Open access
- Published: 09 August 2024
A case report of fludarabine associated ectopic atrial bradycardia and literature review of fludarabine induced bradycardia
- Steve Kong 1 ,
- Sanjana Nagraj 2 ,
- Dennis L. Cooper 3 ,
- Kevin J. Ferrick 2 &
- Lili Zhang 2
Cardio-Oncology volume 10 , Article number: 50 ( 2024 ) Cite this article
Metrics details
Fludarabine is a chemotherapeutic agent with lymphodepleting effects that is increasingly used as part of a conditioning regimen prior to allogeneic stem cell transplantation. Fludarabine is generally considered a relatively safe medication with only rare cases of cardiotoxic side effects.
Case Presentation
Here, we present a case of a 30-year-old woman who was undergoing conditioning for a haploidentical cell transplantation for treatment of Fanconi anemia with a 5-day course of daily fludarabine infusion. After her second fludarabine infusion, she was noted to have ectopic atrial bradycardia that resolved with supportive therapy and completion of fludarabine infusion.
We report the first case of ectopic atrial bradycardia associated with fludarabine. Although rare and transient, clinicians should recognize this rare cardiotoxic side effect of fludarabine.
Fludarabine is a synthetic purine analog that is commonly used in the treatment of hematologic malignancies, including chronic lymphocytic leukemia, multiple myeloma, Waldenstrom’s macroglobulinemia, and non-Hodgkin’s Lymphoma [ 1 ]. Fludarabine is also used as part of lymphodepleting chemotherapy prior to chimeric antigen receptor (CAR) T cell treatment and as a conditioning agent for allogeneic stem cell transplantation where its immunosuppressive properties facilitate engraftment [ 2 ]. The major toxicity of fludarabine is myelosuppression, and a small risk of late myelodysplasia [ 3 ]. Although, cardiotoxicity associated with chemotherapeutic agents such as anthracyclines, 5-fluorouracil, and taxanes is well-documented in the literature, fludarabine is considered relatively safe in respect to its effects on the cardiovascular system, with limited number of case reports documenting myocarditis, congestive heart failure, and arrhythmias [ 4 , 5 , 6 ]. Arrhythmias associated with fludarabine include atrial fibrillation, atrial flutter, supraventricular tachycardia, and sinus bradycardia [ 4 , 7 , 8 ]. Given the paucity of literature on the effects of fludarabine on the cardiovascular system, subtle cardiovascular effects may be missed in clinical practice and under-reported by physicians. Here, we present the first case of fludarabine associated ectopic atrial bradycardia in a young woman undergoing conditioning for haploidentical cell transplantation for the treatment of Fanconi anemia. We also present a review of the current literature on fludarabine induced bradycardia.
Case presentation
A 30-year-old woman with a history of type 2 diabetes was diagnosed with Fanconi Anemia in December 2022. She had immigrated from the Dominican Republic with a history of long-standing anemia and thrombocytopenia. The patient underwent a bone marrow biopsy in August 2022 that showed hypercellular marrow with dysplastic myeloid precursors, absent megakaryocytes, and karyotypic abnormalities consistent with myelodysplastic syndrome (MDS). The patient underwent chromosome breakage studies and then next generation sequencing studies in December 2022 that confirmed the diagnosis of Fanconi anemia with mutations in the FANCA gene. She was admitted to our hospital for chemotherapy and reduced intensity conditioning haploidentical cell transplant including rabbit anti-thymocyte globulin (ATG), fludarabine, and 300 centigray (cGy) total body irradiation. She underwent a transthoracic echocardiogram that showed normal biventricular size and function with a left ventricular (LV) ejection fraction of 60%. The baseline EKG showed normal sinus rhythm (Fig. 1 ).
EKG performed on day 1 of hospital course, prior to ATG and Fludarabine infusions, showing sinus rhythm with ventricular rate of 74
At the time of admission, the patient’s vital signs were within normal limits, blood pressure 114/75 mmHg, heart rate 74 beats per minute (bpm), respiratory rate 18 breaths per minute, and SpO2 100% on room air. During the first three days of her hospital stay, she received daily infusions of rabbit ATG which was associated with fever that resolved with steroids. Subsequently, she was started on a 5-day course of fludarabine infusions at a dose of 30 mg/m 2 per day. However, 12 h after the completion of her second fludarabine infusion, the patient was noted to have a heart rate of 40–50 bpm. EKGs showed evidence of ectopic atrial bradycardia with ventricular rate of 44 bpm (Fig. 2 ), a significant change from her baseline. Other vital signs showed BP 111/74 mmHg, respiratory rate of 18 breaths per minute, and SpO2 of 100% on room air. There were no electrolyte abnormalities noted on her laboratory data. Review of her recent medications did not reveal any agents that could have caused bradycardia. Despite the new onset bradycardia, the patient remained asymptomatic with appropriate chronotropic response with activity, with heart rate that improved to 70–80 bpm with exertion.
EKG performed on hospital day 6, after 2 doses of fludarabine infusions, showing presence of negative P waves on leads II and III representing ectopic P waves and bradycardia with ventricular rate of 44
A transthoracic echocardiogram performed on day 6 of hospital course (3 days after starting fludarabine), showed normal LV size and function with an estimated LV ejection fraction of 60%. It also showed a mildly dilated right ventricle (RV) with a proximal right ventricular outflow tract (RVOT) measurement of 4.1 cm on parasternal long axis view (Fig. 3 a) and a basal diameter 4.2 cm and mid cavity diameter 3.7 cm on apical 4 chamber view along with mild RV dysfunction (Fig. 3 b), which were not previously seen. Decision was made to closely monitor the patient on a Holter monitor and to complete the remaining infusions of fludarabine. A Holter Monitor was used as the Oncology unit did not have the capacity to place her on telemetry. She had a 24-h Holter Monitor placed during hospital day 6, day 3 of fludarabine infusion, that showed increased supraventricular ectopic beats, particularly while the patient was asleep between 5–6 am, 13–14 h after her third fludarabine infusion (Fig. 4 ).
a and b Parasternal long axis and apical 4 chamber views showing mild RV dilation, with a measured proximal RVOT of 4.1 cm [Upper limit normal < 3 cm] and RV basal diameter 4.2 cm and mid cavity diameter 3.7 cm [Upper limit normal < 4.1 cm and < 3.5 cm respectively] with basal RV hypokinesis on TTE performed on hospital day 6, after 2 doses of fludarabine infusions
Holter monitor report of Supraventricular Ectopic Beat Hourly Burden while patient was receiving her third fludarabine infusion on hospital day 6. Patient received the third fludarabine infusion from 13:17 to 13:47.
Subsequently, on day 4 of 5 fludarabine infusion, the heart rate improved to 50–60 bpm at rest. On day 5 of 5 of fludarabine infusion, the patient’s resting heart rate had returned to her baseline of 60–70 bpm. A repeat transthoracic echocardiogram one day after the completion of her fludarabine infusions, showed normal LV size and LV ejection fraction of 65% with return of RV size to normal (proximal RVOT measurement of 2.9 cm on parasternal long axis view and basal diameter 3.1 cm, mid cavity diameter 3.2 cm, and longitudinal diameter 6.2 cm on apical 4 chamber view), and no further evidence of RV dysfunction (Fig. 5 a and b).
a and b Parasternal long axis and apical 4 chamber views showing normal RV size on repeat echocardiogram on hospital day 9, 1 day after completion of fludarabine infusions, with proximal RVOT measurement of 2.9cm [Upper limit normal <3cm] and RV basal diameter 3.1cm and mid cavity diameter 3.2cm [Upper limit normal <4.1cm and <3.5cm respectively]
In this report, we present a case of fludarabine associated ectopic atrial bradycardia in a young woman undergoing conditioning for a haploidentical cell transplantation. Ectopic atrial bradycardia was first observed 12 h after the completion of her second fludarabine infusion. Other causes of bradycardia were excluded, including electrolyte abnormalities, medications, thyroid dysfunction, and ischemia. Patient was noted to have increased supraventricular ectopic beats on a 24-h Holter Monitor, 13–14 h after her third fludarabine infusion. Bradycardia resolved completely by the fifth fludarabine infusion (Fig. 6 ). She remained asymptomatic through her periods of bradycardia with appropriate chronotropic response to activity. The Adverse Drug Reaction Probability Scale (Naranjo) was calculated to be a total score of 2, suggesting that the bradycardia was a possible adverse drug reaction to Fludarabine.
Timeline of events since admission and onset of bradycardia on day 6, 12 h after patient’s second fludarabine infusion. Resolution of bradycardia on day 8 on her last day of fludarabine infusion
We conducted a literature search using MEDLINE, journals and manuscripts deposited in PubMed Central, and National Center for Biotechnology Information. A search using the terms “fludarabine” and “bradycardia” resulted in five search results, while a search using “fludarabine” and “heart rate” resulted in three search results. After excluding overlaps and only including results that specified the type of observed bradycardia, a total of three articles met criteria for review.
First article was a case-report published by Chung-Lo et al. in 2010 that reported a 22-year-old male with acute myelogenous leukemia who developed sinus bradycardia after infusion of fludarabine 30 mg/m 2 [ 9 ]. This patient was scheduled to receive five days of daily infusions of fludarabine. He started experiencing sinus bradycardia at a rate of 45–50 bpm, thirty minutes after the initial fludarabine infusion was started. The patient remained bradycardic until he completed his five-day course of fludarabine.
The second article was a recent retrospective case-series study published by Celik et al. in 2023 that included 73 patients who received fludarabine, of which 13 patients had developed bradycardia [ 8 ]. The median time to develop bradycardia after fludarabine administration was 25 min. No pathological findings other than sinus bradycardia were observed. Notably, the only significant difference between patients who developed bradycardia and those who did not was patient age, with the mean age in the bradycardia group being 19 years younger than those without bradycardia. The median age of patients who experienced bradycardia was 34, and the median age of those without bradycardia was 53.
The third article was a prospective cohort study published by Poreba et al. in 2018 in which 56 patients with hematologic malignancies who were to undergo conditioning with high dose chemotherapy followed by hematopoietic stem cell transplantation (HSCT) were placed on a 24-h EKG monitor prior to therapy followed by a post-HSCT 24-h EKG monitor [ 10 ]. The results showed that post HSCT had a statistically significant higher percentage of premature ventricular contractions, tachycardia, and Mobitz type 1 s degree atrioventricular block. There was no statistically significant difference in the number of patients who experienced bradycardia. Of the 56 patients, 6 patients underwent chemotherapy conditioning with fludarabine. However, it did not disclose which patients with bradycardia had been treated with fludarabine. Additionally, the type of bradycardia the patients experienced was also not mentioned.
There have been several proposed mechanisms of how different chemotherapeutic agents can contribute to bradycardia, including interference with cardiac conduction, hypersensitivity reaction, stimulation of parasympathetic system, direct toxic and negative chronotropic effects, electrolyte disturbances, and transient ischemia [ 11 , 12 , 13 , 14 ]. However, the effect of fludarabine on the cardiovascular system and the mechanism of fludarabine induced bradycardia is not well understood and sparsely reported in the literature. One proposed mechanism suggests that fludarabine is a nucleoside prodrug that has an intermediate metabolite called fluoroadenosine, which may have similar signaling characteristics to adenosine, activating the acetylcholine-gated potassium channel which slows the sinoatrial (SA) node pacemaker rate [ 8 ].
The diagnosis of ectopic atrial bradycardia was derived by the morphology of the P waves on the patient’s EKGs. The P waves in leads II, III, and AVF are negatively vectored and positively vectored in lead V1 (Fig. 2 ). These abnormal P wave vectors are indicative of a P wave that likely originated from outside the SA node. Furthermore, the very short PR intervals on her EKGs also suggest that the P waves are likely originating from outside the SA node. However, another etiology to consider is a junctional escape rhythm with a retrograde atrial activation, in which the retrograde conduction is faster than the antegrade conduction leading to the P wave falling before the QRS complexes.
Importantly, this is the first case of fludarabine associated bradycardia that showed the emergence of an ectopic P wave bradycardia. We hypothesize that when her SA node was slowed by fludarabine, a competing ectopic atrial focus took over the pacing duties, resulting in the ectopic atrial bradycardia that were seen on serial EKGs and the Holter Monitor. Of note, her 24-h Holter Monitor showed that there was an increased presence of supraventricular ectopic beats while the patient was sleeping. This is likely due to the combined effects of fludarabine induced slowing of the SA node and the increased parasympathetic tone during sleep, leading to the ectopic atrial focus to take over as the pacing cells [ 15 ].
Our patient was also noted to have mild RV dilation with mild RV dysfunction after two doses of fludarabine, which was not present in her baseline transthoracic echocardiogram. Interestingly, this RV dysfunction was no longer present on a repeated transthoracic echocardiogram performed one day after the completion of her fludarabine infusion. We hypothesize that this transient RV dysfunction was caused by increased loading conditions from large volume infusions as a part of the chemotherapy protocol. However, direct effects of fludarabine cannot be excluded. Fludarabine induced cardiomyopathy is thought to be very rare with only two articles published on such occurrences. Ritchie et al. reported three patients who developed LV systolic dysfunction 12–16 days after the completion of a 5 day induction of fludarabine infusions at a dose of 150 mg/m 2 per day [ 6 ]. All three patients had recovery of their LV function 29–150 days after infusion. However, Newbery et al. reported a single patient who developed LV dysfunction after four infusions of fludarabine at a dose of 30 mg/m 2 per day. This patient continued to have persistent LV dysfunction six months after completion of her regimen [ 4 ].
It is important to acknowledge that ATG has been associated with episodes of bradycardia. Bradycardia is recognized as a “rare” side effect in the manufacturer’s brochure, with an estimated occurrence of 1.5%. From our literature review of “antithymocyte globulin” and “bradycardia”, we were able to find four case reports (2 cases in children and 2 cases in adults) and a retrospective study in children reporting bradycardia after administration of ATG [ 16 , 17 , 18 , 19 , 20 ]. In all these cases, bradycardia was noted minutes to hours after the initiation of ATG. In this case presentation, however, the temporal relationship between the development of bradycardia and administration of ATG compared to the administration of fludarabine is important. The patient developed bradycardia 12 h after the second fludarabine infusion, which is 3 days after the completion of her ATG infusions. Bradycardia also resolved on the last day of fludarabine infusion. Thus, it seems more likely that bradycardia was secondary to fludarabine, rather than ATG. However, it is possible that ATG may have had a theoretical additive effect with fludarabine. With increased usage of ATG and fludarabine as conditioning agents for haploidentical cell transplantation in patients with sickle cell anemia and aplastic anemia, increased awareness of possible bradycardia with these two medications may be important [ 18 , 19 ].
In general, fludarabine remains a relatively safe medication in respect to its effects on the cardiovascular system, with only a limited number of cases of adverse cardiac events published throughout the literature. Most of these reported cardiovascular adverse reactions are transient and usually do not require any additional therapy. Instead, patients typically have a good prognosis with a full recovery back to their baseline with close monitoring and supportive care [ 6 , 8 , 9 ].
Bradycardia is a relatively rare side-effect of fludarabine. We report the first case of ectopic atrial bradycardia associated with fludarabine. Close monitoring and supportive care are usually indicated. As fludarabine is ubiquitously used for a dramatically increasing number of patients receiving CAR T cell therapy for treatment of lymphoma and myeloma, clinicians should be aware of its potential for cardiac side effects.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
Anti- thymocyte globulin
Beats per minute
Chimeric antigen receptor
Electrocardiogram
Hematopoietic stem cell transplantation
Left ventricular
Myelodysplastic syndrome
Right ventricle
Right Ventricular Outflow Tract
Wright SJ, Robertson LE, O’Brien S, Plunkett W, Keating MJ. The role of fludarabine in hematological malignancies. Blood Rev. 1994;8(3):125–34.
Article PubMed CAS Google Scholar
Lukenbill J, Kalaycio M. Fludarabine: a review of the clear benefits and potential harms. Leuk Res. 2013;37(9):986–94.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88.
Newbery G, Lima NA, Gurgel LA, Driscoll R, Lima CCV. Persistent heart failure following melphalan and fludarabine conditioning. J Cardiol Cases. 2019;20(3):88–91.
Article PubMed PubMed Central Google Scholar
Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced Cardiotoxicity. Maedica (Bucur). 2013;8(1):59–67.
PubMed Google Scholar
Ritchie DS, Seymour JF, Roberts AW, Szer J, Grigg AP. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant. 2001;28(1):101–3.
Peres E, Levine JE, Khaled YA, Ibrahim RB, Braun TM, Krijanovski OI, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(1):149–52.
Çelik S, Güven ZT, Altınsoy A, Tubay Ş, Keklik M, Ünal A. Fludarabine-induced bradycardia in allogeneic hematopoietic stem cell transplantation: A retrospective study. J Oncol Pharm Pract. 2023:10781552231189868.
Chung-Lo W, Hsieh CY, Chiu CF, Bai LY. Fludarabine-induced bradycardia in a patient with refractory leukemia. Hematol Oncol Stem Cell Ther. 2010;3(2):99–100.
Article PubMed Google Scholar
Poręba M, Gać P, Usnarska-Zubkiewicz L, Pilecki W, Kuliczkowski K, Mazur G, et al. The analysis of the parameters of 24-hr ECG Holter monitoring in patients with blood neoplasms undergoing high-dose chemotherapy and stem cell transplantation. Ann Noninvasive Electrocardiol. 2018;23(4):e12534.
Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47.
Yusuf SW, Razeghi P, Yeh ET. The diagnosis and management of cardiovascular disease in cancer patients. Curr Probl Cardiol. 2008;33(4):163–96.
McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273–9.
Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MC, Donehower RC. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9(9):1704–12.
Chouchou F, Desseilles M. Heart rate variability: a tool to explore the sleeping brain? Front Neurosci. 2014;8:402.
Loushin MK, Hasinoff IK, Belani KG. A delayed cardiopulmonary reaction to an intravenous immunosuppressant thymoglobulin after pancreas transplant. Anesth Analg. 2001;93(5):1260–1 table of contents.
Kao SY, Xu W, Brandwein JM, Lipton JH, Messner HA, Minden MD, et al. Outcomes of older patients (> or = 60 years) with acquired aplastic anaemia treated with immunosuppressive therapy. Br J Haematol. 2008;143(5):738–43.
Godown J, Deal AM, Riley K, Bailliard F, Blatt J. Worsening bradycardia following antithymocyte globulin treatment of severe aplastic anemia. J Pediatr Pharmacol Ther. 2011;16(3):218–21.
PubMed PubMed Central Google Scholar
Elazhary S, Alawyat HA. Bradycardia associated with antithymocyte globulin treatment of a pediatric patient with sickle cell disease: a case report and literature review. Hematol Transfus Cell Ther. 2022;44(2):284–7.
Kállay K, Zakariás D, Csordás K, Benyó G, Kassa C, Sinkó J, et al. Antithymocyte Globuline Therapy and Bradycardia in Children. Pathol Oncol Res. 2019;25(2):487–92.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Medicine, NYC Health + Hospitals/North Central Bronx Hospital, Bronx, NY, USA
Division of Cardiology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA
Sanjana Nagraj, Kevin J. Ferrick & Lili Zhang
Division of Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA
Dennis L. Cooper
You can also search for this author in PubMed Google Scholar
Contributions
SK, SN, and LZ collected patient data. SK, SN, LZ, DLC, and KJF interpreted patient data. SK, SN, LZ, and KJF analyzed EKG, echocardiogram, and Holter Monitor. SK, SN, and LZ performed literature reviews of fludarabine and bradycardia. SK and SN wrote the manuscript. DLC, KJF, and LZ critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Steve Kong .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
The patient provided consent and gave permission to have her case, as well as relevant related workup and diagnostic images, presented in the medical literature.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kong, S., Nagraj, S., Cooper, D.L. et al. A case report of fludarabine associated ectopic atrial bradycardia and literature review of fludarabine induced bradycardia. Cardio-Oncology 10 , 50 (2024). https://doi.org/10.1186/s40959-024-00253-x
Download citation
Received : 18 March 2024
Accepted : 29 July 2024
Published : 09 August 2024
DOI : https://doi.org/10.1186/s40959-024-00253-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bradycardia
- Chemotherapy
- Conditioning Agent
- Ectopic atrial bradycardia Fludarabine
Cardio-Oncology
ISSN: 2057-3804
- General enquiries: [email protected]

COMMENTS
Introduction. Aplastic anemia (AA) is a rare condition characterized by the combination of hypoplasia or aplasia of the bone marrow and pancytopenia in at least two of the three main lines of cells: red blood cells (RBCs), white blood cells (WBCs), and platelets [].An estimated incidence of this disease is 0.6 to 6.1/million per year with a sex ratio of about 1:1 [].
Abstract. Establishing a diagnosis of aplastic anemia (AA) can be challenging, but it is absolutely critical to appropriate management, especially differentiating between acquired and inherited forms of the disease. The hematology field requires updated diagnostic guidelines to ensure that appropriate clinical pathways are pursued for patients ...
Patients with aplastic anemia are found to have profound hypocellular bone marrow, but no hemophagocytes should be found. Case study submitted by Tzu-Fei Wang, MD, The Ohio State University, Columbus, OH. Citations. Citations APA. American Society of Hematology. (1). Case Study: 44-Year-Old Man with Fever, Abdominal Pain, and Pancytopenia. ...
Acquired severe aplastic anemia (SAA) is a rare hematologic disease associated with significant morbidity and mortality. Immune destruction of hemopoietic stem cells plays an important role in pathogenesis, as shown by successful treatment with immunosuppressive agents, leading to transfusion independence or complete recovery of peripheral blood counts in a proportion of patients.
In this study, we present cases of two Iranian boys aged 13 and 8 years with hepatitis of unknown origin who developed aplastic anemia in the course of hospitalization. Hepatitis-associated aplastic anemia is a well-known immune-mediated form of aplastic anemia that we detected in our patients and treated with immunosuppressive therapy.
References. Aplastic anemia is a disease with a long history. The first case description was published by Paul Ehrlich in 1888, the term "anemia aplastique" originated with Louis Henri Vaquez ...
Approximately two-thirds of patients do not have a suitable MRD; in that case, ... in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematol-Hematol J ...
Aplastic anemia is an historic disease. The first patient was described by the young Paul Ehrlich in 1885, "anemia aplastique" originated with Vaquez in 1904, and its clinical features were described by Cabot and other pathologists in the early 20 th century. In the modern era, an almost uniformly fatal prognosis, mainly for young persons ...
Aplastic Anemia. A plastic anemia is a disease with a long history. The first case description was published by Paul Ehrlich in 1888, the term "anemia aplas-tique" originated with Louis Henri ...
Dr. Philippe-Antoine Bilodeau (Medicine): A 21-year-old man presented to this hospital with a sore throat, epistaxis, and petechiae of the oropharynx. One week before the current presentation ...
Aplastic anaemia is a rare, previously fatal condition with a significantly improved survival rate owing to advances in understanding of the pathophysiology and improved treatment strategies including haematopoietic stem cell transplantation. Although a rare condition, aplastic anaemia continues to present a high burden for affected patients ...
This patient has severe aplastic anemia (SAA). SAA is most commonly immune-mediated and is defined as having bone marrow cellularity lower than 25 percent and two or more of the following 1 : peripheral blood neutrophil count lower than 0.5 × 10 9 /L; peripheral blood platelet count lower than 20 × 10 9 /L, and peripheral blood reticulocyte ...
Despite the precision of its diagnostic criteria, aplastic anemia has always been a diagnosis of exclusion. No single test allows us to reliably diagnose idiopathic aplastic anemia, but the field has advanced considerably in terms of awareness of and diagnosis of other disorders resulting in a similar or indistinguishable hematologic phenotype. 1-4 Consequently, the diagnostic evaluation has ...
Fanconi anemia is the most common hereditary cause. It presents in the late first decade with pancytopenia, organ hypoplasia, and bone defects including abnormal radii, absent thumbs, and short stature. Seronegative hepatitis is responsible for 5% to 10% of total cases. Telomerase defects are found in 5% to 10% of adult-onset aplastic anemia.
Prior research has identified associations between immune cells and aplastic anaemia (AA); however, the causal relationships between them have not been conclusively established. A two-sample ...
A case-control interview study of aplastic anemia was conducted to evaluate suspected risk factors. Cases (N = 59) newly diagnosed during 1975-82 at 25 Baltimore area hospitals were compared with 59 individually matched (on age, sex and race) controls selected by random digit dialing. The average educational level was less for cases than controls.
Severe aplastic anaemia (SAA) is a rare and life-threatening bone marrow failure disorder. We used data from the transplant outcomes in aplastic anaemia study to characterize mosaic chromosomal alterations (mCAs) in the peripheral blood of 738 patients with acquired SAA and evaluate their associations with telomere length (TL) and survival post-haematopoietic cell transplant (HCT).
Acquired aplastic anemia is caused by immune-mediated destruction of hematopoietic stem and progenitor cells. 1 CD34+ cells and early progenitors are uniformly reduced in aplastic anemia. 2 Bone ...
Aplastic anemia is a term describing the common findings of pancytopenia and marrow hypoplasia arising from a variety of disease states, includin ... In any case, non-transplanted ... an international Fanconi anemia registry study. Blood. 1997; 90: 105 -110. 36. Wagner JE, Tolar J, Levran O, et al. Germline mutations in BRCA2: shared genetic ...
The bone marrow failure (BMF) state of aplastic anemia (AA) is marked by cytopenias and ineffective hematopoiesis. 1 AA confers a significant risk for morbidity and death as a result of its progressivenatural history and/or complications related to suboptimal therapy. 2, 3 Without definitive treatment, mortalityfrom severe AA (SAA) approaches 70% at 2 years. 4 Establishing an accurate etiology ...
According to a cohort study involving 122 patients with severe aplastic anemia, who received ATG with CSA therapy, the recorded response rates were 60%, 61%, and 58% at 3, 6, and 12 months, respectively, after initiation of treatment 9. In this particular case, the response was recorded 18 months after the initial phase of therapy.
Nipocalimab delayed or prevented severe fetal anemia and 54 percent of study participants in the Phase 2 UNITY study achieved a live birth at or after 32 weeks without the need for intrauterine transfusion (IUT) The AZALEA Phase 3 clinical study is currently enrolling patients: Nipocalimab is the only therapy in clinical development for use in pregnancies at risk for severe hemolytic disease ...
Abstract. Aplastic anemia is a rare disease of the hematopoietic system. Although some viral agents have been implicated, the association between COVID-19 and aplastic anemia is unclear. In this way, several cases of aplastic anemia have been reported following infection with COVID-19. Importantly, we reported a 16-year-old girl with severe ...
The second article was a recent retrospective case-series study published by Celik et al. in 2023 that included 73 patients who received fludarabine, of which 13 patients had developed bradycardia ... and fludarabine as conditioning agents for haploidentical cell transplantation in patients with sickle cell anemia and aplastic anemia, ...