- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Forums Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications

How to Perform the Iodine Clock Reaction
Last Updated: January 15, 2024
This article was reviewed by Anne Schmidt . Anne Schmidt is a Chemistry Instructor in Wisconsin. Anne has been teaching high school chemistry for over 20 years and is passionate about providing accessible and educational chemistry content. She has over 9,000 subscribers to her educational chemistry YouTube channel. She has presented at the American Association of Chemistry Teachers (AATC) and was an Adjunct General Chemistry Instructor at Northeast Wisconsin Technical College. Anne was published in the Journal of Chemical Education as a Co-Author, has an article in ChemEdX, and has presented twice and was published with the AACT. Anne has a BS in Chemistry from the University of Wisconsin, Oshkosh, and an MA in Secondary Education and Teaching from Viterbo University. This article has been viewed 50,491 times.
If you are a science teacher who wants to amaze your students, or just an average amateur chemist, then this is the experiment for you! The classic iodine clock reaction demonstrates the properties of chemical kinetics through its mesmerizing change in color, and it is sure to fascinate you and perhaps your audience. With just a few household items, you can easily perform this experiment with great success.

- 10 volume hydrogen peroxide
- 1000 mg vitamin C tablets
- 5% Iodine tincture
- Distilled water
- Containers (preferably clear)
- Coffee filters

Community Q&A
- Your containers can be reused after the experiment. Wash them with a bit of dish soap, and they're good to go! Thanks Helpful 1 Not Helpful 0
- This experiment can easily fail, so don't despair if your first attempt doesn't go as planned. Thanks Helpful 1 Not Helpful 0
- If your vitamin C tablets or iodine tinctures are of a different concentration, then you will need to mathematically scale it to the correct ratio. Thanks Helpful 1 Not Helpful 1

- Iodine tinctures can stain clothing and skin, so avoid getting it on them. Thanks Helpful 1 Not Helpful 0
You Might Also Like

About This Article

- Send fan mail to authors
Did this article help you?

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life

Lab Procedure: Iodine Clock Reaction

Core Concepts
In this lab tutorial, we learn about the iodine clock reaction, including its procedure, underlying chemistry, and data analysis.
Topics Covered in Other Articles
- Lab Safety Rules
- Recrystallization
- Thin Layer Chromatography
- Distillation
- Integrated Rate Laws
What is the Iodine Clock Reaction?
The Iodine Clock Reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry . For this, the reaction persists as a staple of general chemistry lab demonstrations.
In this experiment, you prepare two simple, transparent solutions. Once the solutions combine, however, the mixture gradually turns from clear to dark blue to near-black. This color change corresponds to the progress of the reaction, which allows you to visually witness the kinetics in a way that most reactions do not provide.
Interestingly, some chemists colloquially call this reaction the “Egyptian Night” experiment. In Egypt, the darkness of nighttime often arrives rather suddenly, similar to rapid dark color change in this reaction.


Iodine Clock Procedure
To perform the Iodine Clock Reaction, you need an iodine salt, a reductant, an oxidant, an acid , starch, and water as a solvent. As mentioned before, these components become allocated between three different solutions according to these specifications:
- First Solution: Starch, Water.
- Second Solution: Iodine Salt, Reductant, Water.
- Third Solution: Oxidant, Acid, Water.
Once the solutions mix, the reaction begins.
The most common variant of the Iodine Clock Reaction uses sodium thiosulfate (Na 2 S 2 O 3 ) as the reductant and hydrogen peroxide (H 2 O 2 ) as the oxidant. Potassium iodide (KI) serves as the salt, while sulfuric acid (H 2 SO 4 ) provides the required acidity. Importantly, gloves, safety goggles, and caution should be observed when using sulfuric acid and hydrogen peroxide to prevent chemical burns.
As we’ll find out in a later section, the kinetics of the reaction depends on the concentrations of acid, iodide, and oxidant. Thus, most lab procedures studying reaction kinetics will vary the concentrations of one or more of these species. Aside from that, the reductant concentration tends to be kept low, as very little is required, while the starch tends to be in excess.
The Chemistry of the Iodine Clock
Iodine clock redox and kinetics.
Before the three solutions mix into one, each ionic species dissociates into their respective ions:
KI → K + + I –
Na 2 S 2 O 3 → 2Na + + S 2 O 3 2-
H 2 SO 4 → H + + HSO 4 –
During the reaction, K + , Na + , and HSO 4 – do not participate, remaining as spectator ions. Once the solutions mix, the hydrogen peroxide oxidizes the iodide into diatomic iodine:
2H + + H 2 O 2 + 2I – → I 2 + 2H 2 O
Importantly, as the reaction produces diatomic iodine, the thiosulfate re-reduces the iodine back to iodide:
2S 2 O 3 2- + I 2 → 2I – + S 4 O 6 2-
This back and forth between iodide and iodine continues until all thiosulfate oxidizes away. Afterward, significant quantities of iodide and iodine exist at the same time. They react with one another to form the triiodide ion:
I 2 + I – → I 3 –
This triiodide ion then forms a complex with the starch. This complex is responsible for the increasing dark blue of the reaction vessel. As a side note, due to the striking dark blue of the complex, a mixture of iodine and iodide called Lugol’s iodine is used to test for trace amounts of starch .

Iodine Clock Kinetics
The first reaction, the oxidation, occurs much slower than the reduction, making it the rate-determining step during that first phase of the reaction. Additionally, once the reduction ceases, the oxidation continues to serve as the rate-determining step, as both the formation of the triiodide and the starch complex occur relatively quickly. Thus, for the entirety of the experiment, oxidation determines the progress of the dark blue hue. This is true even though the starch complex is not directly generated from the oxidation.
Aside from the qualitative observation of the increasingly blue reaction vessel, you can periodically measure the starch concentration through spectrophotometry . The resulting data then allows you to quantify the reaction kinetics.
Kinetic Data Analysis
First, you need to do multiple trials of the Iodine Clock with different concentrations of potassium iodide. Then, you quickly place these samples into a spectrophotometer that records concentrations at consistent time intervals. You’d want to set the spectrophotometer to a frequency similar to 600nm to pick up the dark blue of the starch complex.
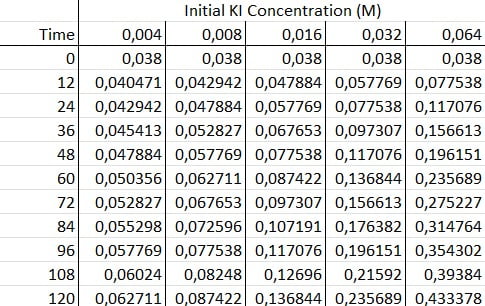
Next, you graph your data. You should find that the absorbance of each graph increases linearly with time. This makes sense since chemical reactions always initially proceed at linear rates.

Finally, to determine the reaction order with respect to KI, you take the logarithms of the initial concentrations and reaction rates and generate a log/log graph.

The slope of the resulting slope corresponds to the reaction order in our rate law , due to the properties of logarithms.
RxnRate = k’[KI] n
log(RxnRate) = log(k’[KI] n ) = nlog([KI]) + log(k’)
k’: Relative rate constant (s -1 )
n: Reaction Order of KI
The graph then generates a trendline of y = x – 1.2883, indicating that the Iodine Clock Reaction is first order with respect to KI (n = 1).
Quick links
- Make a Gift
- Directories
Iodine Clock Reaction
Warning : never let solution B stand in open beaker for over an hour. Test existing stock solutions. If demonstration does not work, discard solution B.
Chemicals and Solutions
- Iodine clock solution A
- Iodine clock solution B
Solution Preparation
0.02 M KIO 3
Solution B
4g soluble starch, 0.2g sodium metabisulfite ( Na ₂ S ₂ O ₅), 5mL 1M sulfuric acid in 1L solution
- Electronic stop clock
- Ice bath in large refrigerator dish
- 100 mL cylinders
- 400 mL beakers
- Magnetic stirrer and stirring bars for both beakers
- Into cylinders, put 100 mL of solution A.
- Into beakers, put 100 mL of solution B.
- Put one cylinder and beaker into ice bath at least 15 minutes before expected use.
- Mix together and observe the color change.
- ALTERNATIVE: to illustrate effect of concentration, put differing amounts of solution A in cylinders and top off with DI water, mixing each cylinder with an equal volume of solution B (e.g. 50 mL solution A with 50 mL DI water mixed with 100 mL solution B). Measure rate of reaction with a stop clock.
A simplified explanation of the reaction is as follows:
I- reacts with IO ₃ - to form I ₂ .
\( \ce{ 5HI_{(aq)} + HIO3_{(aq)} -> 3I2 + 3H2O } \)
I ₂ is immediately consumed by reaction with HSO ₃ -.
\( \ce{ I2 + HSO3- + H2O -> 2I- + SO42-_{(aq)} + 3H+_{(aq)} } \)
When HSO ₃ - has been consumed, I ₂ accumulates. I ₂ + starch forms a blue colored starch-I ₂ complex.
When you dilute solution A (0.02 M KIO ₃ ) in half, it takes twice as long to form the blue starch - I ₂ complex. When solution A is diluted to 1/4 the concentration, it takes four times as long to form the blue starch-I ₂ complex. The reaction is much slower at colder temperatures.
The solutions can be poured down the drain. (The bisulfite and iodate are consumed and the iodine is complexed with starch so there is no oxidizer hazard). Demo generates 800 mL of 0.2% starch iodine complex aq.
Summerlin and Ealy, Chemical Demonstrations , pp. 75-76.
Clock II (Oscillating Clock)
30% hydrogen peroxide is very reactive.
Solution #1
36 mL of 30% H ₂ O ₂ to 100 mL (make fresh)
Solution #2
43 g KIO ₃, 4.3 mL conc. sulfuric acid, in 1L solution
Solution #3
- 15.6 g malonic acid, 3 g of MnSO ₄ in 970 mL of water.
- Stir in 30 mL of a 1% starch solution.
- magnetic stirrer and bar
- Three 100 mL graduated cylinders
- 400 mL beaker
- Measure out 100 mL of each solution into graduated cylinders.
- With stirring, quickly add each solution to the 400 mL beaker. The solution will oscillate between colorless, amber and dark blue.
- Clock will oscillate for about 5 minutes typically.
Hint : When no stir bar is used, regions of the solution will change first.
The oscillations are due to the shifting concentrations of I ₂ and I - . The amber color is due to the presence of I ₂ . When I - is present, it reacts with I ₂ and starch to produce a dark blue complex. This color fades as iodine is consumed.
A very simplified explanation of this reaction is:
\( \ce{ $\underset{\text{gold}}{\ce{ 2HIO3 + 5H2O2 -> I2 + 5O2 + 6H2O }}$ } \)
\( \ce{ I2 + CH2(COOH)2 -> ICH2(COOH)2 + H+ + I- } \)
\( \ce{ $\underset{\text{dark blue}}{\ce{ I2 + I- + starch -> starch-iodine complex }}$ } \)
\( \ce{ $\underset{\text{colorless}}{\ce{ I2 + 5H2O2 -> 2HIO3 + 4H2O }}$ } \)
The hydrogen peroxide and iodate are consumed and the iodine is complexed with starch so there is no oxidizer hazard. Therefore the solution can be rinsed down the drain. Demo generates 300 mL of 2% starch iodine complex aq.
- Newsletter
- News Feed
