- Skip to main content
- Accessibility information

- Enlighten Enlighten

Enlighten Theses
- Latest Additions
- Browse by Year
- Browse by Subject
- Browse by College/School
- Browse by Author
- Browse by Funder
- Login (Library staff only)
In this section
Inflammatory markers as novel predictors of cardiovascular disease
Welsh, Paul I. (2008) Inflammatory markers as novel predictors of cardiovascular disease. PhD thesis, University of Glasgow.
Inflammation is widely considered to be an important contributing factor in atherogenesis and the risk of atherothrombotic complications. Baseline measurements of some inflammatory markers are known to be predictive risk factors for future cardiovascular disease (CVD) events in prospective epidemiological studies. Inflammatory markers dominant in the literature are acute phase response (APR)-associated and include fibrinogen, C-reactive protein (CRP) and, more recently, interleukin- (IL-) 6. This thesis reviews the literature and suggests the need for further research into novel inflammatory markers of CVD risk. The broad aim was to expand on limited existing data and ascertain if circulating levels of four novel inflammatory markers (tumour necrosis factor alpha [TNF alpha], IL-18, soluble CD40 ligand [sCD40L], and matrix metalloproteinase-9 [MMP-9]) are associated with classical cardiovascular risk factors, and with future CVD events in several epidemiological studies.
In studies of pre-analytical variables, all four markers had commercially available assays acceptable for epidemiological use, but only IL-18 and TNF alpha displayed assay stability and the ability to be measured in plasma or serum.
Due to limited serum samples, MMP-9 and sCD40L were less extensively measured. Results suggest a moderate positive association of MMP-9 with coronary heart disease (CHD) risk (although confounded by smoking and markers of general inflammation), while serum sCD40L may be moderately inversely related to CHD risk. More data is required for these markers.
IL-18 and TNF alpha displayed similar degrees of short-term biological variability and regression dilution as CRP. Population distributions of both cytokines were consistent with limited previous reports. Both displayed associations with conventional vascular risk factors (such as age, gender, HDL cholesterol, and smoking), although interestingly, associations with epidemiological measures of obesity were poor. Both cytokines demonstrated moderate associations with vascular disease in a retrospective CHD study. In 3 prospective CHD or CVD studies, IL-18 demonstrated consistent but moderate associations with risk of vascular events in age- and sex-adjusted models (Odds ratio [OR]~1.6 in the top versus bottom third of the population). The association became borderline significant after adjustment for conventional risk markers. Associations of TNF alpha with risk of CHD in these studies were inconsistent, and more data are needed.
In 3 prospective stroke studies, TNF alpha demonstrated some moderate associations with acute stroke outcome and recurrent stroke risk, but not with incident stroke in the elderly with vascular disease. IL-18 demonstrated no association with risk or outcome in any stroke study.
Meta-analysis in 4 suitable prospective studies showed (in full adjustment models) that IL-18 (OR 1.18 [95% CI 0.95-1.48] comparing extreme thirds) and TNF alpha OR 1.05 [0.67-1.64]) have at best weak independent associations with CVD risk. Therefore these markers are unlikely to add significantly to clinical vascular risk prediction models, although these cytokines may still be of biological significance and potential therapeutic targets. More data is required for these markers.
In conclusion IL-18, TNF alpha, MMP-9 and sCD40L may show weak associations with CVD. However, despite animal and tissue models indicating that they may play pivotal roles in atherogenesis, circulating concentrations of these inflammatory markers have limited independent vascular risk associations. Elevated circulating levels of APR-associated markers may sensitively reflect exposure to a wide range of adverse pro-inflammatory stimuli including lifestyle exposures, whereas some other inflammatory markers may not.
Actions (login required)
Downloads per month over past year
View more statistics

The University of Glasgow is a registered Scottish charity: Registration Number SC004401
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Factors Associated with Inflammation Markers, a Cross-Sectional Analysis
Tess v clendenen, karen l koenig, alan a arslan, annekatrin lukanova, franco berrino, goran hallmans, annika idahl, vittorio krogh, anna e lokshin, adele marrangoni, brian m nolen, nina ohlson, roy e shore, sabina sieri, anne zeleniuch-jacquotte.
- Author information
- Article notes
- Copyright and License information
Corresponding Author: Tess Clendenen, Department of Environmental Medicine, Division of Epidemiology, New York University School of Medicine, 650 1st Ave., New York, NY, USA. Phone: 212-263-0365, Fax: 212-263-8570, [email protected]
Issue date 2011 Dec.
Epidemiological studies have reported associations between circulating inflammation markers and risk of chronic diseases. It is of interest to examine whether risk factors for these diseases are associated with inflammation. We conducted a cross-sectional analysis to evaluate whether reproductive and lifestyle factors and circulating vitamin D were associated with inflammation markers, including C-reactive protein, cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, TNFα), and cytokine modulators (IL-1RA, sIL-lRII, sIL-2Ra, sIL-4R, sIL-6R, sTNF-R1/R2), among 616 healthy women. We confirmed associations of several inflammation markers with age and BMI. We also observed significantly higher levels of certain inflammation markers in postmenopausal versus premenopausal women (TNFα, sIL-1RII, sIL-2Ra), with increasing parity (IL-12p40), and with higher circulating 25(OH) vitamin D (IL-13) and lower levels among current users of non-steroidal anti-inflammatory drugs (NSAIDs) (IL-1β, IL-2, IL-10, IL-12p70, and IL-12p40), current smokers (IL-4, IL-13, IL-12p40), and women with a family history of breast or ovarian cancer (IL-4, IL-10, IL-13). Our findings suggest that risk factors for chronic diseases (age, BMI, menopausal status, parity, NSAID use, family history of breast and ovarian cancer, and smoking) are associated with inflammation markers in healthy women.
Keywords: C-Reactive Protein, Cross-sectional Studies, Cytokines, Cytokine Receptors, Epidemiologic Factors
1. Introduction
Several studies have shown that elevated inflammation markers, primarily C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNFα), are associated with increased risk of cardiovascular disease, type II diabetes, and other chronic conditions, including cancer [ 1 – 9 ]. We recently conducted a case-control study of ovarian cancer nested in three prospective cohorts, and found that IL-2, IL-4, IL-6, IL-12, and IL-13 were associated with risk [ 10 ]. Several inflammation-related markers, including IL-2, IL-5, TNFβ, interferon γ (IFNγ), ICAM, soluble IL-2 receptor (sIL-2R), and soluble TNFα receptor 1 (sTNF-R1), have been found to be positively, and IL-13 inversely, associated with subsequent risk of non-Hodgkin lymphoma (NHL) [ 11 – 13 ]. Given the potential role of inflammation in chronic disease, it is of interest to identify factors that contribute to differences in levels of inflammation markers among healthy people.
The emphasis of previous studies has generally been on the association between a limited number of inflammation markers (usually CRP, IL-6, TNFα) and general lifestyle and/or cardiovascular risk factors [ 1 , 14 , 15 ]. The focus of the present study was to assess whether reproductive and lifestyle factors and circulating vitamin D, which is immunomodulatory and has anti-inflammatory properties in vitro [ 16 , 17 ], are associated with CRP, cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, TNFα) and cytokine modulators/soluble receptors (IL-1 receptor antagonist (Ra), soluble (s)IL-1RII, sIL-2Ra, sIL-4R, sIL-6R, and sTNF-R1/R2) in healthy women. We selected inflammation markers that showed adequate temporal reproducibility (ICC ≥ 0.5) over a 2–3 year period in preliminary reproducibility studies, thus suggesting that a single inflammation marker measurement can be used to rank women according to their average level [ 18 – 20 ].
2. Materials and Methods
2.1 study subjects.
For these cross-sectional analyses, we included healthy controls from two nested case-control studies in which inflammation markers have been measured: 1) a study of ovarian cancer in three prospective cohorts: the New York University Women’s Health Study (NYUWHS-OVCA), the Northern Sweden Health and Disease Study (NSHDS), and ORDET in Italy [ 10 ]; and 2) a study of non-Hodgkin lymphoma in the NYUWHS cohort (NYUWHS-NHL) [ 11 ]. The parent cohorts have been described in detail previously [ 21 – 24 ]. Controls were selected to match cases on age (± 6 months), date of blood sampling (± 3 months), and parent cohort (NYUWHS, NSHDS, or ORDET). Controls also had to be alive and free of cancer at the time of the matched case’s cancer diagnosis. Participants were not eligible for inclusion if they were using hormone replacement therapy (HRT) or oral contraceptives (OC) at the time of blood sampling. Up to two controls per case were selected at random from cohort members who met the above criteria. We included all 616 controls from the nested case-control studies in the present cross-sectional analysis (163 controls from NYUWHS-OVCA, 82 from ORDET, 187 from NSHDS, and 184 from NYUWHS-NHL).
Self-reported questionnaires were used to collect data on lifestyle and reproductive variables and height and weight for the NYUWHS and NSHDS subjects. ORDET participants completed in-person interviews with a nurse who also measured height and weight. Although questionnaires varied according to cohort, harmonization of variables was usually straightforward. Participants were considered to have a family history of breast or ovarian cancer only if cancer was reported for a first degree relative (i.e. mother, sister, or daughter). The NSHDS questionnaire asked about “current use” of NSAIDs whereas the NYUWHS questionnaire asked about NSAID use over the previous four weeks. To harmonize NYUWHS and NSHDS data, we restricted NSAID use to use the day of, or one day prior to, blood sampling for the NYUWHS participants, which is more similar to the data that was collected from NSHDS participants. This cut-off is also justified because the half-life of most NSAIDs is less than two days in circulation [ 25 ]. ORDET did not collect information on NSAIDs.
2.2 Laboratory Methods
Luminex multiplex bead-based technology was used for measurement of inflammation markers in serum (NYUWHS and ORDET) and EDTA plasma (NSHDS) [ 26 ]. The assay kits and procedures and coefficients of variation (CVs) have been described previously in our reproducibility and case-control studies [ 10 , 11 , 19 , 20 ].
25(OH)D was measured using a direct, competitive chemiluminescence immunoassay (DiaSorin LIAISON 25 OH Vitamin D TOTAL Assay) for the NYUWHS-OVCA and NYUWHS-NHL serum samples. NSHDS plasma 25(OH)D was measured using a gamma-B 25-hydroxy vitamin D radioimmunoassay (Immunodiagnostic Systems, Inc.). Details about the 25(OH)D measurements have been described previously [ 27 – 29 ].
2.3 Statistical Methods
Cytokine measurements that were below the lower limit of detection (LLD) were imputed. For eleven markers with less than 5% of the measurements below the lower limit of detection (LLD), we assigned a value equal to the midpoint between the LLD and zero. For seven markers with 5% or more values below the LLD (IL-1β, IL-2, IL-4, IL-5, IL-12p70, IL-13, IL-1RA had 17–25% values below the lower limit of detection), we used a maximum likelihood estimation procedure developed by Lubin et al. to perform multiple imputation in the presence of detection limits [ 30 ]. For the NHL study, sIL-1RII and sIL-4R were not measured, sIL-2Ra was measured with a different assay, and sIL-6R measurements were outside of the detection limits of the assay, thus NHL samples are not included in the analysis of these four markers. Cytokine values were log-transformed to reduce departures from the normal distribution.
We estimated geometric means for each cytokine adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, NSHDS, ORDET), age (continuous), and BMI (continuous, log 2 -transformed) within categories of a number of descriptive variables: age (adjusted for study and BMI only), BMI (adjusted for study and age only), menopausal status (pre/post), number of full term pregnancies, smoking status at blood sampling (current/former/never), first degree family history of breast or ovarian cancer (yes/no), use of NSAIDS at blood sampling (yes/no), use of vitamin supplements at time of blood sampling (yes/no), and ever use of OCs (yes/no). Multivariate regression analyses were conducted to assess which variables were independently associated with each cytokine. Partial Pearson correlations were estimated between log-transformed cytokines and cytokine modulators, adjusting for study, age and BMI. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC). All statistical tests were two-sided and P -values < 0.05 were considered significant.
The Institutional Review Board of New York University School of Medicine, the Ethical Review Board of the National Cancer Institute of Milan (Italy), the Regional Ethical Committee of the University of Umeå, Sweden, and the Swedish Data Inspection Board reviewed and approved this study.
Characteristics of the study subjects are shown in Table 1 . The age range at blood sampling was 30–75 years (mean = 55 years), and 66% of the women were post-menopausal. The median BMI was about 25kg/m 2 . Thirty-five percent of women had previously used oral contraceptives, 23% were nulliparous, and 82% of parous women had 2 or more children. Eighteen percent of women reported having a first-degree family history of breast or ovarian cancer. At the time of blood sampling, 18% were current smokers, 17% were taking NSAIDs, and 42% were using multivitamins.
Descriptive Characteristics of Study Subjects
Note: NSAIDs: non-steroidal anti-inflammatory drugs, 25(OH)D: 25-hydroxy vitamin D
High proportion of missing data because this variable was not available from ORDET (n=82) subjects.
High proportion of missing data because this variable was not available from ORDET (n=82) subjects and from subjects who were not included in previous studies on 25(OH)D.
Cytokine Distributions by Subject Characteristics
Age-, BMI-, and study-adjusted geometric means and 95% confidence intervals (CI) for selected characteristics are shown in Tables 2 and 3 for cytokines and cytokine modulators, respectively. Table 4 shows beta estimates from multivariate models controlling for all characteristics significantly associated with each marker.
Cytokines: Adjusted Geometric Means and 95% Confidence Intervals Among Categories of Selected Characteristics
Note: NSAIDs: non-steroidal anti-inflammatory drugs, 25(OH)D: 25-hydroxy vitamin D, CRP: C-reactive protein, IL: interleukin, TNF: tumor necrosis factor All cytokine units are pg/mL unless otherwise specified.
Number of women for whom the biomarker was above the LLD (fewer women may be included in analyses of characteristics with missing data).
Model adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, ORDET, NSHDS) and BMI
Model adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, ORDET, NSHDS) and age at sampling
Models adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, ORDET, NSHDS), age at sampling, and BMI
P-value for trend among parous women
Cytokine Modulators: Adjusted Geometric Means and 95% Confidence Intervals Among Categories of Selected Characteristics
Note: NSAIDs: non-steroidal anti-inflammatory drugs, 25(OH)D: 25-hydroxy vitamin D, IL: interleukin, RA: receptor antagonist, Ra: receptor alpha, R: receptor, TNF: tumor necrosis factor All cytokine units are pg/mL.
sIL-1RII and sIL-4R were not measured in the NYUWHS-NHL study (n=184 subjects). Because sIL-2Ra was measured in the NYUWHS-NHL study using a different manufacturer’s kit and protocol, the NYUWHS-NHL subjects are not included for this marker. sIL-6R values were above the upper limit of detection for 90% of the NYUWHS-NHL samples, thus the NHL subjects are not included for this marker.
Multivariate Regression Coefficient Estimates for Factors Independently Associated with Cytokines and Cytokine Modulators
Note: NSAIDs: non-steroidal anti-inflammatory drugs, CRP: C-reactive protein, IL: interleukin, Ra: receptor alpha, R: receptor, TNF: tumor necrosis factor
All models adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, ORDET, NSHDS); BMI was log 2 -transformed, cytokines and cytokine modulators were log 2 +1 transformed, and age, BMI, and inflammation markers were modeled on the continuous scale unless otherwise noted. IL-1RA and sIL-4R are not included in the table because there were no significant predictors.
Models adjusted for menopausal status are also adjusted for age even if it was not statistically significant.
Models evaluating smoking status include a categorical variable with levels for current, former, and never smokers (as shown in Tables 2 and 3 ), though betas are only shown for the associations that were statistically significant.
The association with BMI was significant for BMI ≥ 30kg/m 2 vs. BMI < 30kg/m 2 , but not for BMI on the continuous log scale.
Age and Menopausal Status
There was a significant increasing trend across age groups for CRP, IL-6, and TNFα ( Table 2 ) and for sIL-1RII, sIL-2Ra, sIL-6R, sTNF-R1, and sTNF-R2 ( Table 3 ). After adjusting for menopausal status ( Table 4 ), age was no longer significantly associated with TNFα, sIL-1RII, and sIL-2Ra; these three markers were significantly higher in postmenopausal women ( Tables 2 – 4 ).
As shown in Tables 2 and 3 , age-adjusted inflammation marker levels increased significantly with increasing BMI for five markers: CRP, TNFα, sIL-1RII, sIL-6R, and sTNF-R1. Though other cytokines did not show a significant trend with increasing BMI, levels were highest among obese women (BMI > 35 kg/m 2 ) for several markers.
IL-12p40 showed a significant increasing trend with parity ( Table 3 ), though this association was no longer significant after adjustment for smoking ( Table 4 ).
Family History of Breast or Ovarian Cancer
Several markers were significantly lower in women with a family history of breast or ovarian cancer: IL-4, IL-10, and IL-13 ( Table 2 ). However, the association between family history of breast and ovarian cancer was no longer significant for IL-4 or IL-13 after controlling for smoking ( Table 4 ).
Current use of NSAIDs
Current users of NSAIDs had significantly lower levels of IL-1β, IL-2, IL-10, IL-12p70, and IL-12p40 ( Tables 2 and 3 ). However, NSAID use was no longer associated with IL-12p40 after controlling for smoking ( Table 4 ).
Smoking Status
Current smokers had significantly lower levels of IL-4, IL-13, and IL-12p40 ( Tables 2 – 4 ). Former smokers had significantly lower levels of IL-4 and IL-10 ( Table 2 ) and higher levels of sTNF-R2 ( Table 3 ). However, IL-10 was not associated with smoking in multivariate models, while IL-6 was lower among former smokers in the multivariate model adjusted for age ( Table 4 ).
Vitamin D (25(OH)D)
Women with 25(OH)D levels above 50 nmol/L had generally higher levels of inflammatory markers than did women with lower 25(OH)D levels, but the difference was only statistically significant for IL-13 ( Tables 2 and 3 ), and this difference was no longer significant after adjustment for smoking ( Table 4 ).
Other Variables
We did not observe any significant differences in cytokine levels related to ever use of oral contraceptives or current use of multivitamins. Thus, these characteristics are not shown in Tables 2 – 4 .
Cytokine Correlations
Pearson correlation coefficients adjusted for age, BMI, and study are shown in Table 5 . CRP was weakly correlated with the cytokine IL-6 and the cytokine modulator sIL-2Ra (r~ 0.1–0.2). Cytokines were significantly correlated with all other cytokines. The strongest correlations (r > 0.6) were observed between IL-1β and IL-2, IL-10 and IL-12p70, and among the following markers: IL-4, IL-5, IL-6, and IL-13. Correlations between cytokines and cytokine modulators were weak for most pairs (r <0.4), though moderate correlations (r~0.5) were observed between IL-12p40 and two modulators: IL-1RAandsTNF-R1.
Adjusted Pearson Correlation Coefficients for Cytokines and Cytokine Modulators a
Note: CRP: C-reactive protein, IL: interleukin, RA: receptor antagonist, Ra: receptor alpha, R: receptor, TNF: tumor necrosis factor. Cytokines and cytokine modulators were log transformed.
Coefficients with * are significant at P < 0.01, with ** at P <0.001, and with *** at P <0.0001.
Correlations are based on a different number of subjects for each pair of markers, ranging from 390 to 616 subjects.
All models adjusted for study (NYUWHS-OVCA, NYUWHS-NHL, ORDET, NSHDS), age at blood sampling, and body mass index.
4. Discussion
Our results support three previous studies that observed higher levels of the pro-inflammatory cytokine TNFα among post-menopausal women [ 31 – 33 ], although others did not observe such a difference [ 34 , 35 ], or observed lower levels [ 36 ]. Two soluble cytokine receptors, sIL-1RII and sIL-2Ra were also higher among post- vs. pre-menopausal women. The associations were apparent after adjustment for age and BMI, which suggests that these markers may be influenced by sex hormones.
Increasing parity was associated with higher levels IL-12p40, although this association was no longer statistically significant after adjustment for smoking. We are unaware of previous reports that have evaluated the association between previous pregnancies and inflammation markers. However, a microarray study of normal breast tissue found that several inflammation-associated genes were upregulated in both recently (0–2 years since pregnancy) and distantly (5–10 years since pregnancy) parous vs. nulliparous women [ 37 ]. However, despite adjustment for age and BMI, we cannot exclude the possibility that the association may have been confounded by other factors, including age at each pregnancy and time since last pregnancy.
Women with a first degree family history of breast or ovarian cancer had lower levels of several cytokines. While inflammation marker levels are not likely to be directly related to family history, this risk factor may be associated with adoption of healthy lifestyle elements.
Current use of NSAIDs was inversely associated with a number of inflammatory markers (IL-1β, IL-2, IL-10 and IL-12p70), in line with our expectations. However, NSAID use was not associated with levels of CRP. Prior reports on the association between NSAID use and CRP are conflicting [ 38 – 43 ], which may be due to differences in duration, types, and doses of NSAID evaluated, but also because some studies were limited in sample size [ 38 – 40 , 42 ], included subjects with existing chronic disease [ 43 ], or reported associations for regular users who were asked to abstain from use prior to blood sampling [ 41 ].
Somewhat contrary to our expectations, smoking status was not significantly associated with CRP, IL-6, TNFα or other pro-inflammatory cytokines. In our study, current smokers generally had lower levels of cytokines than non-smokers, though these associations were only statistically significant for the anti-inflammatory markers, IL-4, IL-13 and IL-12p40. Lower levels of these cytokines among smokers vs. non-smokers were observed in all four sub-studies (NYUWHS-OVCA, NYUWHS-NHL, ORDET, and NSHDS). Previous studies on current smoking and cytokines among healthy individuals have found positive [ 34 , 44 – 50 ], inverse [ 51 , 52 ], and null [ 49 , 52 – 55 ] associations. Inconsistent findings may be a result of incomplete control for lifetime exposure to cigarette smoke (e.g. pack-years), which may be a more relevant measure of exposure than current smoking status.
CRP was not correlated with most cytokines. Given the role of IL-6, and to a lesser extent TNFα and IL-1β, in the induction of CRP, we expected to observe moderate to strong correlations of CRP with these cytokines. In models adjusted for study and age (but not BMI), CRP was positively correlated with TNFα (r = 0.1, P < 0.005) and IL-6 (r= 0.1, P=0.001), but not IL-1β. Others have found a moderate correlation between CRP and IL-6, some of which adjusted for BMI, with most estimating a correlation coefficient between 0.3–0.5 [ 2 , 14 , 34 , 41 , 50 , 56 , 57 ]. However, data from the Women’s Health Initiative trial suggests that there could be IL-6-independent pathways for regulating CRP, as CRP and IL-6 had different associations with several cardiovascular risk factors: HRT use, alcohol use, and exercise [ 44 ]. For example, the consistent association between CRP and HRT [ 58 ] vs. the weak or null association between IL-6 and HRT, may be due to direct (IL-6-independent) hepatic induction of CRP [ 44 ].
The observed significant correlation between TNFα, IL-6, and IL-1β was expected due to the regulatory inter-relationship of these cytokines [ 34 , 41 , 56 , 59 , 60 ]. Cytokines were correlated with each of the other cytokines, and less so with cytokine modulators. In physiological conditions, elevations in pro-inflammatory cytokines trigger elevations in anti-inflammatory cytokines to resolve the inflammatory response. Thus, we expected all pro- and anti-inflammatory cytokines to be positively correlated with each other in healthy women. However, since all the cytokines were measured using the same assay kit, we cannot rule out the possibility that imperfect antibody specificity could have contributed to the strength of the correlations. The relationship between cytokines and their modulators is complex, because soluble cytokine receptors can act as both cytokine agonists and/or antagonists [ 61 ]. Possible reasons for the lack of correlation between cytokines and their modulators have been suggested by others, such as the longer half-life of soluble receptors in circulation and/or the inability to detect all unbound and bound forms of cytokines with immunoassays [ 62 – 64 ].
The lack of association between cytokines and 25(OH)D, the best representation of an individual’s vitamin D status, is in agreement with a study of vitamin D supplementation in overweight and obese individuals that did not find an association between circulating 25(OH)D and CRP or cytokines (including IL-2, IL-4, IL-5, IL-10, IL-12, IL-13) [ 52 ]. We found some evidence of a positive association for IL-13 and 25(OH)D, though the association was not significant in multivariate analyses. Others have reported a lack of association between 25(OH)D and several inflammation markers (CRP, IL-1β, IL-6, IL-10, SIL-2R, TNFα, sTNF-R1, and sTNF-R2) [ 65 – 70 ], though one small study found an inverse association between 25(OH)D and TNFα [ 65 ] and two others found that vitamin D supplementation stabilized [ 71 ] or reduced [ 72 ] TNFα among patients with congestive heart failure or obesity, respectively. We note that the median 25(OH)D level in our study group (median: 48 nmoI/L, interquartile range: 25, 81 nmoI/L) is considered to be in the “insufficient” range (~<50 nmoI/L) based on some recommendations [ 73 , 74 ].
This study has a number of strengths. First, participants were healthy and not using HRT or OCs for at least 6 months prior to blood sampling, thus minimizing the effects of existing disease and exogenous hormones on cytokine levels. Second, we evaluated associations between risk factors and a large number of cytokines and cytokine modulators which have not been evaluated in previous studies. Third, all inflammation marker measurements were performed in the same laboratory, which minimizes assay variability and allows individuals to be ranked relative to others.
Our study also has some limitations. First, questionnaires differed between cohorts. However, results were usually consistent across cohorts, providing confidence in our findings. Second, it is possible that measured levels of a single cytokine are not reflective of their biologically relevant concentration. This may be due to assay limitations in quantification of absolute levels and/or the presence of circulating cytokine inhibitors, including soluble receptors and other binding proteins. Finally, the exploratory nature of our analyses resulted in a substantial number of statistical tests, thus it is likely that some associations were significant due to chance. However, we did not adjust for multiple comparisons because our goal was to identify general patterns of association that may explain risk factor associations and to aid in the selection of potential confounders for future studies of cytokines and chronic disease risk. The associations observed here should be evaluated in independent studies.
In conclusion, we observed the expected relationships between inflammation markers and age and BMI. Higher levels of certain markers were observed among postmenopausal vs. premenopausal women (TNFα, sIL-1RII, and sIL-2Ra) and with increasing parity (IL-12p40). Lower levels were observed among current versus non-users of NSAIDs (IL-10, IL-β, IL-10, IL-12p70, and IL-12p40), women with a family history of breast or ovarian cancer (IL-4, IL-10, and IL-13), and current versus non-smokers (IL-4, IL-13, and IL-12p40). These findings suggest that one mechanism underlying the relationship between reproductive and lifestyle factors and chronic diseases may involve inflammation mediators.
Highlights.
We assessed potential determinants of inflammation markers among 616 healthy women.
Observed associations between lifestyle and reproductive factors and inflammation markers.
Associations: age, BMI, menopause, parity, NSAIDs, family history of cancer, smoking.
Acknowledgments
This work was supported by research grants from the National Cancer nstitute (R21 CA116585, R01 CA098661, and P30CA016087) and the National nstitute of Environmental Health Sciences Center Grant (ES000260) at the National nstitutes of Health.
Abbreviations
25-hydroxyvitamin D
body mass index
coefficient of variation
confidence interval
C-reactive protein
diet and hormones in the etiology of cancer study
ethylenediaminetetraacetic acid
hormone replacement therapy
inter-cellular adhesion molecule
interleukin
interleukin-1 receptor antagonist
intra-class correlation coefficient
lower limit of detection
metabolic equivalent tasks
New York University Women’s Health Study
non-Hodgkin lymphomas
non-steroidal anti-inflammatory drug
Northern Sweden Health and Disease Study
oral contraceptives
peripheral blood mononuclear cell
soluble IL-1 receptor
soluble IL-2 receptor alpha
soluble IL-4 receptor
soluble IL-6 receptor
tumor necrosis factor
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- 1. Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119(2):166, e17–28. doi: 10.1016/j.amjmed.2005.06.057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. doi: 10.1001/jama.286.3.327. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ridker PM, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–72. doi: 10.1161/01.cir.101.15.1767. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Siemes C, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24(33):5216–22. doi: 10.1200/JCO.2006.07.1381. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Il’yasova D, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Chaturvedi AK, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28(16):2719–26. doi: 10.1200/JCO.2009.27.0454. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Tsilidis KK, et al. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123(5):1133–40. doi: 10.1002/ijc.23606. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Aleksandrova K, et al. Circulating C-Reactive Protein Concentrations and Risks of Colon and Rectal Cancer: A Nested Case-Control Study Within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172(4):407–18. doi: 10.1093/aje/kwq135. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. McSorley MA, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007;109(4):933–41. doi: 10.1097/01.AOG.0000257126.68803.03. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Clendenen TV, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(5):799–810. doi: 10.1158/1055-9965.EPI-10-1180. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Gu Y, et al. Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: a prospective study. Cancer Causes Control. 2010;21(8):1323–33. doi: 10.1007/s10552-010-9560-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Saberi Hosnijeh F, et al. Plasma cytokines and future risk of non-Hodgkin lymphoma (NHL): a case-control study nested in the Italian European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1577–84. doi: 10.1158/1055-9965.EPI-09-1237. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Purdue MP, et al. Prediagnostic serum levels of cytokines and other immune markers and riskofnon-hodgkin lymphoma. Cancer research. 2011;71(14):4898–907. doi: 10.1158/0008-5472.CAN-11-0165. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Danesh J, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5(4):e78. doi: 10.1371/journal.pmed.0050078. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Yamagishi S, et al. Decreased high-density lipoprotein cholesterol level is an independent correlate of circulating tumor necrosis factor-alpha in a general population. Clin Cardiol. 2009;32(9):E29–32. doi: 10.1002/clc.20517. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Cantorna MT, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–79. doi: 10.1016/j.ecl.2010.02.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Wong HL, et al. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Gu Y, et al. Reproducibility of serum cytokines and growth factors. Cytokine. 2009;45(1):44–9. doi: 10.1016/j.cyto.2008.10.014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Clendenen TV, et al. Temporal reliability of cytokines and growth factors in EDTA plasma. BMC Res Notes. 2010;3:302–310. doi: 10.1186/1756-0500-3-302. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Toniolo PG, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer nst. 1995;87(3):190–7. doi: 10.1093/jnci/87.3.190. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Toniolo PG, et al. Endogenous hormones and breast cancer: a prospective cohort study. Breast Cancer Res Treat. 1991;18(Suppl 1):S23–6. doi: 10.1007/BF02633522. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Hallmans G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort - evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Berrino F, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88(5):291–6. doi: 10.1093/jnci/88.5.291. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Rovensky J, Payer J. Dictionary of Rheumatology. Springer; Vienna: 2009. Non-steroidal anti-inflammatory drugs (NSAIDs) - clinical effect and indication; pp. 145–146. [ Google Scholar ]
- 26. Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243(1–2):243–55. doi: 10.1016/s0022-1759(00)00238-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Zheng W, et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):70–80. doi: 10.1093/aje/kwq118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Purdue MP, et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–69. doi: 10.1093/aje/kwq117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Arslan AA, et al. Circulating vitamin d and risk of epithelial ovarian cancer. J Oncol. 2009;2009:672492. doi: 10.1155/2009/672492. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Lubin JH, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–6. doi: 10.1289/ehp.7199. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Sites CK, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77(1):128–35. doi: 10.1016/s0015-0282(01)02934-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Hong SC, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14(5):835–40. doi: 10.1097/GME.0b013e31802cddca. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Kamada M, et al. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol. 2001;184(3):309–14. doi: 10.1067/mob.2001.109940. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Welsh P, et al. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. Br J Haematol. 2008;141(6):852–61. doi: 10.1111/j.1365-2141.2008.07133.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Yasui T, et al. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56(4):396–403. doi: 10.1016/j.maturitas.2006.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Cioffi M, et al. Cytokine pattern in postmenopause. Maturitas. 2002;41(3):187–92. doi: 10.1016/s0378-5122(01)00286-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Asztalos S, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3(3):301–11. doi: 10.1158/1940-6207.CAPR-09-0069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Ikonomidis I, et al. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100(8):793–8. doi: 10.1161/01.cir.100.8.793. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Feng D, et al. Effect of short-term aspirin use on C-reactive protein. J Thromb Thrombolysis. 2000;9(1):37–41. doi: 10.1023/a:1018644212794. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Feldman M, et al. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J Am Coll Cardiol. 2001;37(8):2036–41. doi: 10.1016/s0735-1097(01)01289-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Kim S, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68(1):323–8. doi: 10.1158/0008-5472.CAN-07-2924. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Azar RR, et al. Effects of aspirin (325 mg/day) on serum high-sensitivity C-reactive protein, cytokines, and adhesion molecules in healthy volunteers. Am J Cardiol. 2003;92(2):236–9. doi: 10.1016/s0002-9149(03)00549-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Ho GY, et al. Antagonistic effects of aspirin and folic acid on inflammation markers and subsequent risk of recurrent colorectal adenomas. J Natl Cancer nst. 2009;101(23):1650–4. doi: 10.1093/jnci/djp346. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Bermudez EA, et al. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. ArteriosclerThromb Vase Biol. 2002;22(10):1668–73. doi: 10.1161/01.atv.0000029781.31325.66. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Danesh J, et al. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J. 1999;20(13):954–9. doi: 10.1053/euhj.1998.1309. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Bazzano LA, et al. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann ntern Med. 2003;138(11):891–7. doi: 10.7326/0003-4819-138-11-200306030-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Levitzky YS, et al. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis. 2008;201(1):217–24. doi: 10.1016/j.atherosclerosis.2007.12.058. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Noakes PS, Holt PG, Prescott SL. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy. 2003;58(10):1053–8. doi: 10.1034/j.1398-9995.2003.00290.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Cozen W, et al. Thl and Th2 cytokines and IgE levels in identical twins with varying levels of cigarette consumption. J Clin Immunol. 2004;24(6):617–22. doi: 10.1007/s10875-004-6247-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Pine SR, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer nstitute. 2011;103(14):1112–22. doi: 10.1093/jnci/djr216. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Tsunoda M, et al. Serum cytokine levels, cigarette smoking and airway responsiveness among pregnant women. Int Arch Allergy Immunol. 2003;130(2):158–64. doi: 10.1159/000069008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Jorde R, et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50(2):175–80. doi: 10.1016/j.cyto.2009.12.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Frohlich M, et al. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24(14):1365–72. doi: 10.1016/s0195-668x(03)00260-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Tracy RP, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. ArteriosclerThromb Vase Biol. 1997;17(10):2167–76. doi: 10.1161/01.atv.17.10.2167. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Ahonen TM, et al. Gender difference among smoking, adiponectin, and high-sensitivity C-reactive protein. Am J Prev Med. 2008;35(6):598–601. doi: 10.1016/j.amepre.2008.09.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Spranger J, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–7. doi: 10.2337/diabetes.52.3.812. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Hu FB, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Ridker PM, et al. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100(7):713–6. doi: 10.1161/01.cir.100.7.713. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Pantsulaia I, et al. Genetic and environmental influences on IL-6 and TNF-alpha plasma levels in apparently healthy general population. Cytokine. 2002;19(3):13846. doi: 10.1006/cyto.2002.1959. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Yasui T, et al. Associations of interleukin-6 with interleukin-lbeta, interleukin-8 and macrophage inflammatory protein-lbeta in midlife women. Cytokine. 2008;41(3):302–6. doi: 10.1016/j.cyto.2007.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64(2):135–46. doi: 10.1002/jlb.64.2.135. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Jung T, et al. Enhancement of human IL-4 activity by soluble IL-4 receptors in vitro. Eur J Immunol. 1999;29(3):864–71. doi: 10.1002/(SICI)1521-4141(199903)29:03<864::AID-IMMU864>3.0.CO;2-T. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Mitsuyama K, et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36(1):45–9. doi: 10.1136/gut.36.1.45. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Kovacs E. Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed Pharmacother. 2001;55(7):391–6. doi: 10.1016/s0753-3322(01)00079-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J nflamm (Lond) 2008;5:10–18. doi: 10.1186/1476-9255-5-10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Michos ED, et al. Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or C-reactive protein in the old order amish. Calcif Tissue nt. 2009;84(3):195–202. doi: 10.1007/s00223-008-9209-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Oelzner P, et al. Relationship between soluble markers of immune activation and bone turnover in postmenopausal women with rheumatoid arthritis. Rheumatology (Oxford) 1999;38(9):841–7. doi: 10.1093/rheumatology/38.9.841. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Gannage-Yared MH, et al. Effects of a short-term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post-menopausal women. J Endocrinol nvest. 2003;26(8):748–53. doi: 10.1007/BF03347358. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Vilarrasa N, et al. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010;38(2):235–42. doi: 10.1007/s12020-010-9379-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Shea MK, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167(3):313–20. doi: 10.1093/aje/kwm306. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Schleithoff SS, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Zittermann A, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Lips P. Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol. 2004;89–90(1–5):611–4. doi: 10.1016/j.jsbmb.2004.03.040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Dawson-Hughes B, et al. Estimates of optimal vitamin D status. Osteoporos nt. 2005;16(7):713–6. doi: 10.1007/s00198-005-1867-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (362.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression
Affiliations.
- 1 UT Center of Excellence on Mood Disorder, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, USA; Bipolar Disorder Program, Laboratory of Molecular Psychiatry, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil; Graduation Program in Psychiatry and Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
- 2 Bipolar Disorder Program, Laboratory of Molecular Psychiatry, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil; Graduation Program in Psychiatry and Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
- 3 Interdisciplinary Laboratory of Clinical Neurosciences, Department of Psychiatry, Federal University of São Paulo, São Paulo, SP, Brazil.
- 4 UT Center of Excellence on Mood Disorder, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, USA; Laboratory of Neurosciences, Graduate Program in Health Sciences, Health Sciences Unit, University of Southern Santa Catarina, Criciuma, SC, Brazil.
- 5 Graduation Program in Psychiatry and Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
- 6 UT Center of Excellence on Mood Disorder, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, USA; Bipolar Disorder Program, Laboratory of Molecular Psychiatry, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil; Graduation Program in Psychiatry and Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil. Electronic address: [email protected].
- PMID: 26544749
- DOI: 10.1016/S2215-0366(15)00309-0
Background: Studies investigating inflammatory markers in post-traumatic stress disorder (PTSD) have yielded mixed results. The aim of our study was to compare concentrations of inflammatory markers in patients with PTSD compared with healthy controls.
Methods: We did a meta-analysis and meta-regression of studies comparing inflammatory markers between patients with PTSD and healthy controls by searching PubMed, Embase, Scopus, Web of Science, and PsycINFO for articles published between Jan 1, 1960, and April 7, 2015. From eligible studies (ie, cross-sectional studies or baseline data from longitudinal studies of peripheral blood cytokine concentrations that compared adults with PTSD with healthy controls), we extracted outcomes of interest, such as mean and SD of peripheral blood cytokines, the time of day blood was collected, whether the study allowed patients with comorbid major depressive disorder in the PTSD group, whether patients were medication free, and severity of PTSD symptoms. We undertook meta-analyses whenever values of inflammatory markers were available in two or more studies. A random-effects model with restricted maximum-likelihood estimator was used to synthesise the effect size (assessed by standardised mean difference [SMD]) across studies.
Findings: 8057 abstracts were identified and 20 studies were included. Interleukin 6 (SMD 0.88; p=0.0003), interleukin 1β (SMD 1.42; p=0.045), and interferon γ (SMD 0.49; p=0.002) levels were higher in the PTSD group than in healthy controls. Subgroup meta-analysis of patients who were not given medication showed higher tumour necrosis factor α (TNFα; SMD 0.69, 95% CI 0.35-1.02; p<0.0001) in the PTSD group than the control group in addition to the aforementioned cytokines. TNFα (SMD 1.32, 0.13-2.50; p=0.003), interleukin 1β (SMD 2.35, 0.01-4.68; p=0.048), and interleukin 6 (SMD 1.75, 0.97-2.53; p<0.0001) levels remained increased in the PTSD group in a subgroup meta-analysis of studies that excluded comorbid major depressive disorder. Illness duration was positively associated with interleukin 1β levels (b=0.33, p<0.0001) and severity with interleukin 6 (b=0.02, p=0.042). A model composed of several variables-presence of comorbid major depressive disorder, use of psychotropic medications, assay used, and time of day blood was collected-explained the large amount of heterogeneity between interleukin 1β, interleukin 6, and C-reactive protein studies. Egger's linear regression test revealed a potential publication bias for interleukin 1β. Additionally, for most inflammatory markers, study heterogeneity was reported to be high (I(2)>75%).
Interpretation: PTSD is associated with increased interleukin 6, interleukin 1β, TNFα, and interferon γ levels. This information might be useful for consideration of chronic low-grade inflammation as a potential target or biomarker in PTSD treatment. Use of psychotropic medication and presence of comorbid major depressive disorder were important moderators that might explain the inconsistency between results of previous studies. Our search strategy used a range of databases and we made exhaustive effort to acquire data by contacting the authors. Notably, high levels of between-study heterogeneity were recorded for most cytokine variables measured in our analysis. However, meta-regression analysis could explain a large amount of this heterogeneity.
Funding: None.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Publication types
- Meta-Analysis
- Research Support, Non-U.S. Gov't
- Systematic Review
- Biomarkers / blood*
- Inflammation / blood*
- Regression Analysis
- Stress Disorders, Post-Traumatic / blood*
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Clinical significance of inflammatory markers for evaluating disease severity of mixed-pathogen bloodstream infections of both Enterococcus spp. and Candida spp.
Xueyi shang, yonggang wang.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected]
Corresponding author. [email protected]
Xin Wang, Ming Li and Yang Yang contributed equally to this work and share first authorship.
Received 2023 Apr 8; Revised 2024 Feb 17; Accepted 2024 Feb 21; Collection date 2024 Mar 15.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
In recent decades, there has been a notable increase in the morbidity and mortality rates linked to bacteremia and candidemia. This study aimed to investigate the clinical significance of inflammatory markers in assessing the disease severity in critically ill patients suffering from mixed-bloodstream infections (BSIs) due to Enterococcus spp. and Candida spp.
In this retrospective research, patients diagnosed with BSIs who were admitted to the intensive care unit (ICU) during the period of January 2019 to December 2022 were analyzed. The patients were divided into two groups: a mixed-pathogen BSI group with both Enterococcus spp. and Candida spp., and a single-pathogen BSI group with only Enterococcus spp. The study examined the differences in inflammatory marker levels and disease severity, including Acute Physiology and Chronic Health Evaluation (APACHE) II scores, duration of ICU stay, and 30-day mortality, between the two groups. Furthermore, we sought to scrutinize the potential associations among these aforementioned parameters.
The neutrophil-to-lymphocyte ratios (NLRs) and levels of plasma C-reactive protein (CRP), interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) in the mixed-pathogen BSI group were higher than those in the single-pathogen BSI group. Spearman's rank correlation analysis showed that NLRs and plasma CRP and IL-6 levels were positively correlated with disease severity in the mixed-pathogen BSI group. Further, the levels of plasma IL-8 and TNF-α were also positively correlated with ICU stay duration and 30-day mortality. In multivariate analysis, plasma CRP and IL-6 levels were independently associated with 30-day mortality.
Mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. may give rise to increased NLRs and plasma CRP, IL-6, IL-8, and TNF-α levels in comparison to BSI caused by Enterococcus spp. only, thus leading to elevated disease severity in critically ill patients.
Keywords: Bloodstream infection, Disease severity, 30-Day mortality, C-reactive protein, Pro-inflammatory cytokine

1. Introduction
Enterococcus spp. are a diverse group of lactic acid-fermenting cocci bacteria that are Gram-positive [ 1 ]. The classification of Enterococcus spp. is primarily based on the host and host environment. The most prevalent species, E . faecalis and E. faecium , are commonly found in the human intestinal tract, whereas E. mangii is typically found in natural environments [ 2 ]. Since the 1980s, there has been a rise in the prevalence of multi-drug-resistant strains of E. faecalis and E. faecium , leading to opportunistic infections in patients admitted to the intensive care unit (ICU) worldwide, posing a considerable threat to the health of hospitalized patients [ 3 ]. Based on the previous research findings [ 4 ], it has been established that over half of the clinical isolates of E. faecalis display resistance towards vancomycin, ampicillin, aminoglycosides, and macrolides. The occurrence of Enterococcus infections is typically observed in immunocompromised and critically ill patients who have potential risk factors such as advanced age, diabetes, malignant tumors, heart disease, transplantation, and surgery [ 5 ]. Enterococcus spp. can lead to various infections such as pelvic infection, neonatal infection, urinary tract infection, bloodstream infection (BSI), infectious endocarditis, and other diseases [ 6 ].
Fungi are widely distributed in nature, with approximately 400 species capable of causing various diseases in humans. However, it is worth noting that infections commonly arise from only 30 of these species [ 7 ]. The prevalence of these organisms has notably risen among ICU patients due to frequent invasive examinations and treatment with hormones, anti-tumor drugs, and immunosuppressants, leading to the occurrence of opportunistic or invasive infections [ 8 ]. A prior investigation revealed a notable disparity in the prevalence of fungal infection between the ICU and other hospital wards, with the mortality rate for invasive fungal infection ranging from 40 to 60% [ 9 ]. Candida spp., Aspergillus spp., Cryptococcus spp ., and Mucor spp. are among the commonly encountered opportunistic fungal pathogens in clinical settings [ 10 ]. Enterococcus spp. and certain types of fungi are typically found in the human body as part of the normal flora. However, in patients with compromised immunity, these pathogens can cause opportunistic infections, often resulting in fatalities. The most severe condition among them is sepsis, with approximately 25%–30% of sepsis cases being caused by BSIs [ 11 ]. Sepsis is currently defined as a potentially life-threatening dysfunction of bodily organs in an individual, which is triggered by the person's abnormal response to an infection [ 12 ]. In China, E. faecalis and C. albicans are the most prevalent enterococcal and fungal pathogens, respectively, to cause co-infection in patients with sepsis [ 13 ]. Co-infections by these two pathogens can form mixed-species biofilms at infection sites, leading to increased resistance against antibacterial drugs and macrophages and posing significant challenges in the treatment [ 14 ]. Moreover, studies have demonstrated that the presence of biofilms shields lipoteichoic acid (derived from Enterococci ), which in turn stimulates inflammation in host cells [ 15 ].
BSIs are associated with the highest mortality rate, especially among ICU patients [ 16 ]. A 4-year statistical study conducted in Romania from 2017 to 2020 found that the E. faecalis positivity rate in the blood cultures from the ICU was 6.8%, second only to Coagulase-negative Staphylococci , Klebsiella pneumoniae , Methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii [ 17 ]. Candida spp. is the most prevalent invasive fungus in critically ill patients, and the incidence of candidemia in the ICUs is approximately 10–20 times more than that in general wards [ 16 ]. An epidemiological study reported that Candida spp. was responsible for 22% of hospital-acquired BSIs, surpassing all other pathogens [ 18 ]. Notably, multiple studies have verified that BSIs caused by mixed pathogens (bacteria and Candida spp.) lead to higher morbidity and mortality compared to those caused by monomicrobial or polybacterial pathogens [ 19 ]. Inflammatory responses are common in critically ill patients with severe sepsis or septic shock, reflecting the host's unbalanced response to infection and its relation to morbidity and mortality [ 20 ]. This has also been observed in BSIs caused by a combination of bacterial and Candida spp. infection. Pathogenic bacteria in the bloodstream release toxins and metabolites, thus provoking a systemic inflammatory response [ 21 ]. On the other hand, candidemia is known for its highly invasive nature, which results in the massive secretion of inflammatory mediators and the recruitment of inflammatory neutrophils and monocytes [ 22 ]. From a broader perspective, it is particularly important to monitor the inflammatory response in mixed-pathogen BSIs caused by bacteria and Candida spp., paying particular attention to the link between inflammatory markers and the disease severity.
Bacterial or fungal infections cause an increase in the counts of neutrophils, and hence an increase in neutrophil-to-lymphocyte ratios (NLRs) may be observed in isolated cases. NLR is considered a reliable marker for the diagnosis of bacteremia and sepsis and has a potential value in assessing the severity of sepsis [ 23 ]. The diagnostic value of procalcitonin (PCT) and C-reactive protein (CRP) as inflammatory markers for the early detection of BSIs has been widely recognized. Nevertheless, the use of PCT and CRP for diagnosing fungemia remains controversial [ 24 ]. In addition, pro-inflammatory cytokines, including interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α), have been shown to predict the severity of sepsis in ICU patients [ 25 , 26 ]. These cytokines are also released by host cells in response to Candida albicans and play a crucial role in activating immune effector cells against invading microorganisms [ 27 ]. Patients with candidemia have been found to exhibit significantly elevated levels of these pro-inflammatory cytokines compared to healthy subjects [ 28 ]. Regrettably, there is a limited number of studies that have examined the correlation between levels of inflammatory markers and disease severity in critically ill patients with concurrent fungal and bacterial BSIs. Additionally, no specific strains have been investigated in these studies. Based on the aforementioned research, we proposed that inflammatory markers, including white blood cell (WBC) counts, NLRs, and plasma PCT, CRP, IL-6, IL-8, and TNF-α levels might be of clinical significance in assessing the disease severity in critically ill patients with mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp.
This study aimed to investigate the clinical significance of inflammatory markers in assessing disease severity in BSIs caused by mixed pathogens, specifically the simultaneous presence of Enterococcus spp. and Candida spp. We analyzed the association between levels of inflammatory markers and Acute Physiology and Chronic Health Evaluation (APACHE) II scores, ICU stay duration, and 30-day mortality. To the best of our knowledge, this is the first study to explore this aspect.
2. Materials and methods
2.1. study population.
The study enrolled critically ill patients admitted to the ICU with BSIs caused by Enterococci from January 2019 to December 2022. Critically ill patients were defined as those who exhibited unstable vital signs, rapidly changing conditions, and more than two organ systems functioning in an unstable state that could potentially endangers their lives at any time. BSI was determined by identifying microorganisms that were isolated from blood culture. Candidemia was defined based on criteria established by the Infectious Diseases Society of America [ 29 ]. The criteria for considering a blood culture positive were as follows: there must be at least one positive result from a blood culture, and subsequent blood cultures for the same patient should also yield positive results, indicating the presence of a single infection strain [ 30 ]. This study included patients who met the following inclusion criteria: (1) age ≥25 years and (2) Enterococcus BSI with or without concurrent Candida BSI as determined by blood culturing results. Further, the exclusion criteria were as follows: (1) the existence of other bacterial co-infections, (2) severely immunocompromised, and (3) receiving corticosteroid treatment. Severely immunocompromised patients were defined as individuals who met one or more of the following criteria: (1) a history of using antineoplastic drugs or immunosuppressants within 3 months prior to ICU admission, (2) the presence of hematologic malignancies, and (3) a diagnosis of acquired immunodeficiency syndrome. These exclusions were necessary in order to maintain the integrity and validity of the findings.
2.2. Classification
Depending on the presence of BSI caused by Candida spp, patients were categorized into two groups: (1) the single-pathogen BSI group, comprising patients without Candida BSI; and (2) the mixed-pathogen BSI group, encompassing patients with BSI caused by both Enterococcus spp. and Candida spp.
2.3. Data collection
Clinical data and laboratory results of all included patients in the study were retrospectively collected using the hospital information system (HIS). The integrated medical records during hospitalization can be queried at any time through HIS [ 31 ]; the records are regularly maintained by the staff of the hospital information department, thus avoiding the data to be missed. The main patient characteristics were age, sex, presence of major diseases, BSI classification, the foci of infection, invasive treatment received, antimicrobial treatment received, and blood culture time to positivity. The identification of the infected organ or tissue was established as the foci of infection when the clinical symptoms of bacteraemia appeared and a blood culture was obtained [ 32 ]. This study followed the convention of documenting the focus as uncertain if two or more potential foci were considered equally plausible [ 33 ]. The antibiotic treatment data obtained in this study mainly involve two aspects: the number of antibiotics used and inappropriate initial antimicrobial therapy. Inappropriate initial antimicrobial therapy was characterized as the antibiotic treatment administered within 72 h following the suspicion of BSI that was found to be ineffective against the particular pathogen determined via culture and in vitro susceptibility testing [ 34 ]. Prior to receiving the blood culture results, the administration of antibiotics as a form of empirical treatment did led to a percentage of patients receiving inappropriate initial antimicrobial therapy. Initiation of antimicrobial therapy was required if the patient had a temperature of >38.0 °C or <36.0 °C, experienced chills, or had a systolic blood pressure <90 mmHg [ 35 ]. The disease severity was defined based on APACHE II scores, ICU stay duration, and 30-day mortality. All patients underwent APACHE II score assessment within the first 24 h after admission to the ICU. BSIs were categorized as community-acquired or hospital-acquired infections. Community-acquired BSIs were identified from a positive blood culture collected within 48 h of hospital admission.
Blood samples for cultures were collected from patients presenting with a high fever and suspicion of BSI on at least 3 occasions. The samples for bacterial blood culture were divided into two bacterial culture bottles, each containing 8–10 mL of blood. The anaerobic bottle was inoculated first, followed by the aerobic bottle. For fungal blood culture, the samples were placed in fungal culture bottles, with each bottle containing 3–5 mL of blood. The time interval between the collection of samples for bacterial blood culture and fungal blood culture was controlled within 5 min. The BACT/ALERT-3D automatic blood culture system (BD Medical) was employed to conduct microbial culturing. Bacteraemia is characterized by the occurrence of bacteria in the bloodstream, as evident through the isolation of bacteria from blood cultures. Fungemia refers to the presence of fungi, specifically yeasts from the Candida spp., in the bloodstream [ 12 ]. The identification of Candida was accomplished through the utilization of colony coloration and the Vitek2 YST identification card (Biomerieux), whereas the ATB microbial identification and drug sensitivity analysis system (Biomerieux) was utilized for identifying bacterial species. The susceptibility test of Enterococcus spp. adhered to the guidelines stated in the 2017 version of the Clinical and Laboratory Standards Institute (CLSI) for the interpretation of susceptibility pattern [ 36 ]. The guidelines provided by CLSI were also followed for conducting the testing on Candida spp. The interpretation of susceptibility was based on the definitions outlined in the CLSI editions, specifically CLSI M27-S4 and CLSI M27-S3 [ 37 ].
At our hospital, which is a specialized center for hematology and oncology, the detection of inflammatory factors, including IL-6, IL-8, and TNF-α, is a routine practice in the clinical laboratory. Furthermore, in cases with infectious diseases, especially patients with sepsis, the levels of inflammatory markers are used as reference indicators for evaluating infection control in ICUs at our hospital. PCT, CRP, IL-6, IL-8, and TNF-α levels in blood samples that were collected at the same time as for culturing were analyzed using a SEBIA automatic enzyme-linked immunoassay detector. The upper reference ranges for plasma PCT, CRP, IL-6, IL-8, and TNF-α levels in healthy participants were 0.5 ng/mL, 5 mg/L, 5.9 pg/mL, 62 pg/mL, and 8.1 pg/mL, respectively. Normal reference intervals were acquired following the guidelines provided in the instruction manual of the Immulite 1000 automated chemiluminescence immunoassay analyzer (Siemens) kit. The establishment and verification of these intervals were conducted in adherence to the specifications outlined in the CLSI C28-A3 document [ 38 ]. Besides, WBC counts and NLRs were also analyzed as inflammatory markers. Following admission to the ICU, blood samples were collected on a daily basis and the complete blood cell counts were assessed using a Sysmex XT-2000i automatic five-category analyzer specialized in blood cell analysis. For the purpose of this study, the levels of inflammatory markers obtained on the day of collecting positive blood culture samples of Enterococcus spp. were utilized for analysis.
2.4. Statistical analysis
The Chi-squared test was applied to analyze categorical variables, and their presentation was expressed as numbers (percentages). The statistical analysis of parametric and non-parametric (natural log-transformed) continuous variables in this study was conducted using the two-sample t -test and the two-tailed Mann-Whitney test, respectively. All continuous variables are expressed as means (standard deviations). Survival curves were prepared to determine the dynamics of 30-day survival and compared using the log-rank (Mantel-Cox) test. Spearman's rank correlation analysis was employed to assess the associations between levels of inflammatory markers and disease severity. Additionally, in order to examine the association with 30-day mortality in detail, multivariate logistic regression analysis was conducted, incorporating age, number of major diseases, number of antibiotics used, inappropriate initial antimicrobial therapy received, the foci of infection, and all inflammatory markers included in this study as variables. p -values of less than 0.05 were deemed to be statistically significant, and odds ratios (OR) together with their corresponding 95% confidence intervals (CIs) were reported. Statistical analyses were conducted using GraphPad Prism 8.0.2 software.
In the preliminary screening list, a total of 158 patients with initial and secondary infections caused by Enterococcus spp. were included. A total of 30 patients were excluded due to the presence of other bacterial co-infections, while 19 and 15 patients were excluded due to their immunocompromised status and the administration of corticosteroids during ICU admission, respectively. The final study cohort consisted of 94 patients, who were classified into two categories: individuals solely infected with Enterococcus spp. (n = 43; single-pathogen BSI group) and individuals concurrently infected with Enterococcus spp. and Candida spp. (n = 51; mixed-pathogen BSI group) ( Fig. 1 ).
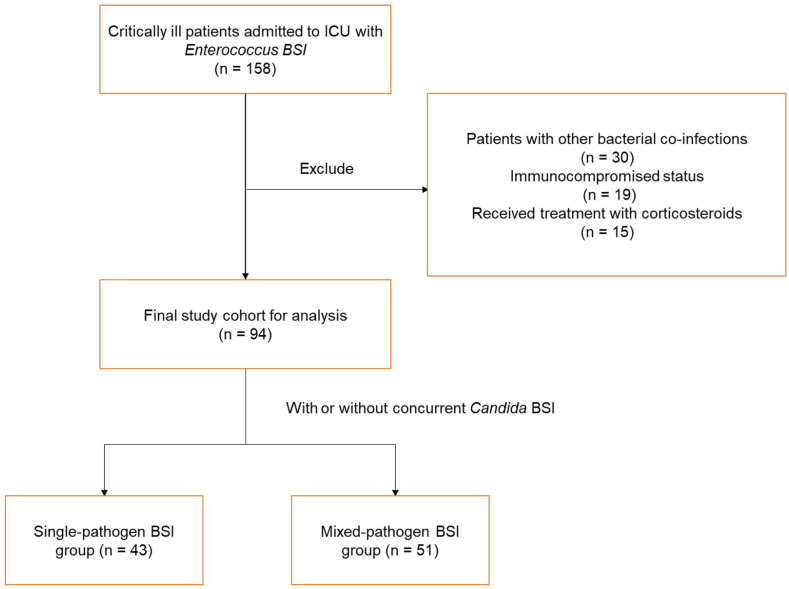
Flow chart showing the inclusion and exclusion criteria.
3.1. Demographic characteristics of included patients
Table 1 summarizes the demographic characteristics of included patients. Single- and mixed-pathogen BSI groups had 43 (45.7%; 30 men and 13 women; the mean age of 75.6 years) and 51 (54.3%; 35 men and 16 women; the mean age of 77.2 years) patients, respectively. Major diseases in patients of these two groups were cardiovascular (41.9% vs. 47.0%), digestive (16.3% vs. 19.6%), urinary system (16.3% vs. 23.5%) and neurological (34.9% vs. 37.2%) diseases, and diabetes mellitus (53.5% vs. 56.8%). The majority of patients had hospital-acquired BSIs in both the single and mixed pathogen BSI groups (53.5% vs. 58.8%). Regarding the foci of infection, in the single-pathogen BSI group, 10 (23.2%) patients had lower respiratory tract infections, 3 (6.9%) had urinary infections, and 14 (32.5%) had gastrointestinal tract infections. Additionally, 16 (37.2%) patients had no certain foci of infection. On the other hand, in the mixed-pathogen BSI group, 13 (25.4%) patients had lower respiratory tract infections, 5 (9.8%) had urinary infections, and 15 (29.4%) had gastrointestinal tract infections. Moreover, 18 (35.3%) patients had no certain foci of infection. All patients in the two groups had a central venous catheter and an indwelling urethral catheter. Further, 31 (72.1%), 5 (11.6%), and 2 (4.6%) patients in the single-pathogen BSI group and 43 (84.3%), 8 (15.7%), and 6 (11.8%) patients in the mixed-pathogen BSI group had indwelling nasogastric, indwelling thoracic drainage, and indwelling peritoneal drainage tubes, respectively. In the single-pathogen BSI group, 8 patients (18.6%) received inappropriate initial antimicrobial therapy, while in the mixed-pathogen BSI group, 15 patients (29.4%) received inappropriate initial antimicrobial therapy. There were no significant differences observed in age, sex, major diseases, BSI classification, primary infected foci, invasive treatment received, and inappropriate initial antimicrobial therapy received between the two groups (all p >0.05). However, the proportion of patients using 3 or more antibiotics in the single-pathogen BSI group was found to be significantly lower than that in the mixed-pathogen BSI group (65.1% vs. 88.2%, respectively; p = 0.012). Additionally, the blood culture time (in hours) to positivity for Enterococcus spp. in the single-pathogen BSI group was significantly shorter than in the mixed-pathogen BSI group [22.8 (7.6) vs. 27.6 (8.4), respectively; p = 0.005]. Furthermore, Candida spp. exhibited a longer blood culture time to positivity compared to Enterococcus spp.
Patient demographics of the final study cohort.
Data are presented as mean (SD) or n (%). APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; ICU, intensive care unit; n, number; SD, standard deviation. For the analysis, the ICU hospitalization days of patients who died within 30 days were also considered as part of the ICU stay duration. p -value < 0.05 means statistically significant. BSIs were community-acquired or hospital-acquired.
3.2. Distribution of pathogens
As depicted in Fig. 2 , the included patients exhibit a varying distribution of pathogens. In the single-pathogen BSI group, it was found that 37 patients were infected with E. faecalis , while 6 patients were infected with E. faecium . On the other hand, in the mixed-pathogen BSI group, 41 patients were found to be infected with E. faecalis , with an additional 10 patients infected with E. faecium . Among these cases, 33 patients displayed co-infections of E. faecalis with C. albicans , 5 patients with C. tropicalis , and E. faecalis , 3 patients with C. glabrata . Furthermore, all of the remaining 10 patients were all co-infected with E. faecium and C. albicans . Notably, no significant differences were observed in the distribution of Enterococcus spp. between the two groups ( p = 0.585).
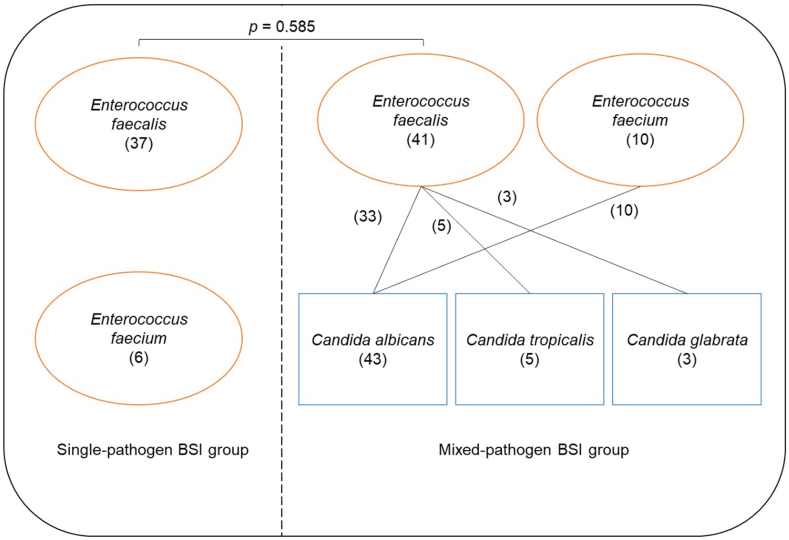
Distribution of pathogens in the two groups. The numbers in parentheses represent the number of cases.
3.3. Disease severity
In Table 1 , the disease severity in both groups is also presented. Within 24 h of ICU admission, APACHE II scores were determined for all 94 patients. Comparing the two groups, the mixed-pathogen BSI group had significantly higher APACHE II scores compared to the single-pathogen BSI group [22.1 (3.7) vs. 19.9 (4.0), respectively; p = 0.002]. In addition, ICU stay duration (in days) was significantly longer for the mixed-pathogen BSI group compared to the single-pathogen BSI group [23.6 (4.7) vs. 20.3 (5.2), respectively; p = 0.005]. The 30-day mortality in the mixed-pathogen BSI group was found to be significantly higher compared to the single-pathogen BSI group (45.1% vs. 23.3%, respectively; p = 0.032). The survival curves of the two groups indicated that deaths in the mixed-pathogen BSI group peaked at day 10, while in the single-pathogen BSI group, deaths were evenly distributed and occurred later. The percent 30-day survival also differed significantly between the two groups (log-rank p = 0.018) ( Fig. 3 ).
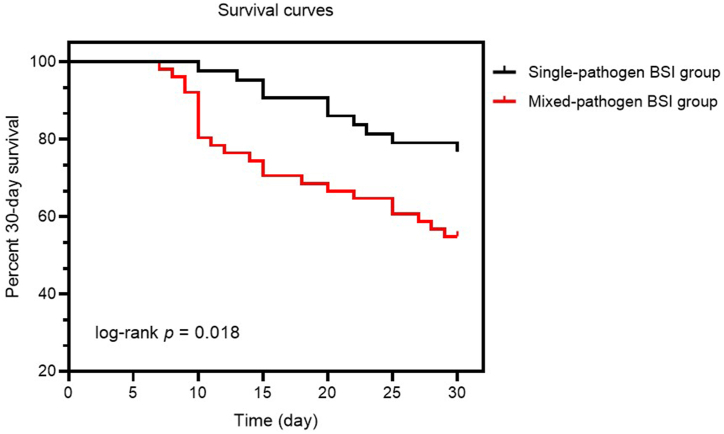
Thirty-day survival curves for patients in two groups. Black and red lines represent 30-day survival curves for the single- and mixed-pathogen BSI groups, respectively. p < 0.05 indicates statistical significance.
3.4. Inflammatory markers
Fig. 4 illustrates the comparisons made between the two groups in terms of WBC counts, NLRs, and plasma PCT, CRP, IL-6, IL-8, and TNF-α levels. The NLRs of the mixed-pathogen BSI group were found to be significantly higher than those of the single-pathogen BSI group ( p = 0.009) ( Fig. 4 b). Additionally, the mixed-pathogen BSI group exhibited significantly higher levels of plasma CRP ( Fig. 4 d), IL-6 ( Fig. 4 e), IL-8 ( Fig. 4 f), and TNF-α ( Fig. 4 g) compared to the single-pathogen BSI group ( p = 0.012, p = 0.003, p = 0.006, and p = 0.026, respectively). However, no statistically significant differences were observed between the two groups in terms of WBC ( Fig. 4 a) counts and plasma PCT ( Fig. 4 c) levels (all p >0.05).
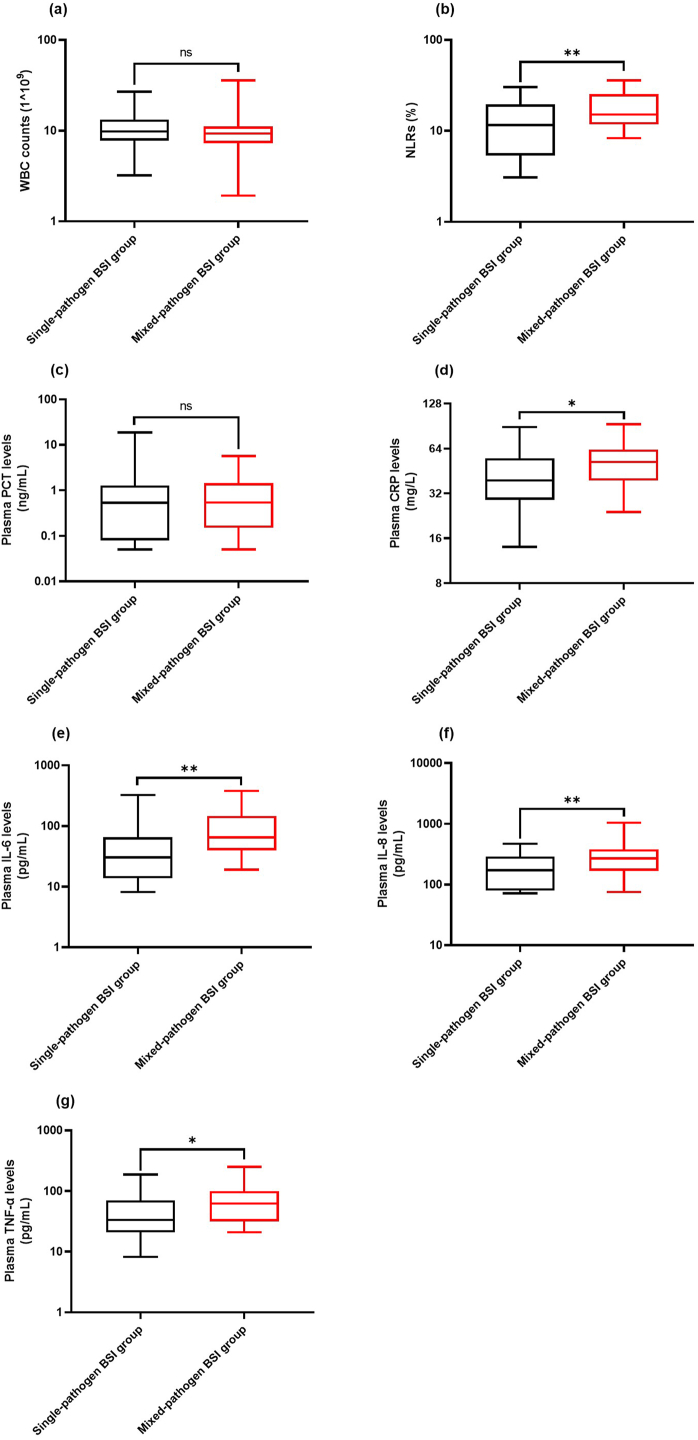
Comparison of inflammatory marker levels between the two groups. Inflammatory markers include WBC counts (a), NLRs (b), plasma PCT levels (c), plasma CRP levels (d), plasma IL-6 levels (e), plasma IL-8 levels (f), and plasma TNF-α levels (g). * and ** represent p < 0.05 and p < 0.01, respectively.
3.5. Association of inflammatory markers with disease severity
Fig. 5 presents the association between inflammatory markers and disease severity in the mixed-pathogen BSI group. NLRs ( Fig. 5 b) and plasma CRP ( Fig. 5 d) and IL-6 ( Fig. 5 e) levels were positively correlated with APACHE II scores [( r = 0.352 and p = 0.038), ( r = 0.436 and p = 0.014), and ( r = 0.413 and p = 0.019), respectively], ICU stay duration [( r = 0.379 and P = 0.025), ( r = 0.405 and p = 0.024), and ( r = 0.506 and p = 0.003), respectively], and 30-day mortality [( r = 0.400 and p = 0.017), ( r = 0.445 and p = 0.012), and ( r = 0.477 and p = 0.004), respectively]. Further, plasma IL-8 ( Fig. 5 f) and TNF-α ( Fig. 5 g) levels also showed a positive correlation with ICU stay duration [( r = 0.392 and p = 0.027) and ( r = 0.362 and p = 0.041), respectively] and 30-day mortality [( r = 0.461 and p = 0.013) and [( r = 0.383 and p = 0.025), respectively]. Nevertheless, WBC counts ( Fig. 5 a) and plasma PCT levels ( Fig. 5 c) exhibited no significant correlation with APACHE II scores, ICU stay duration, and 30-day mortality (all p >0.05).
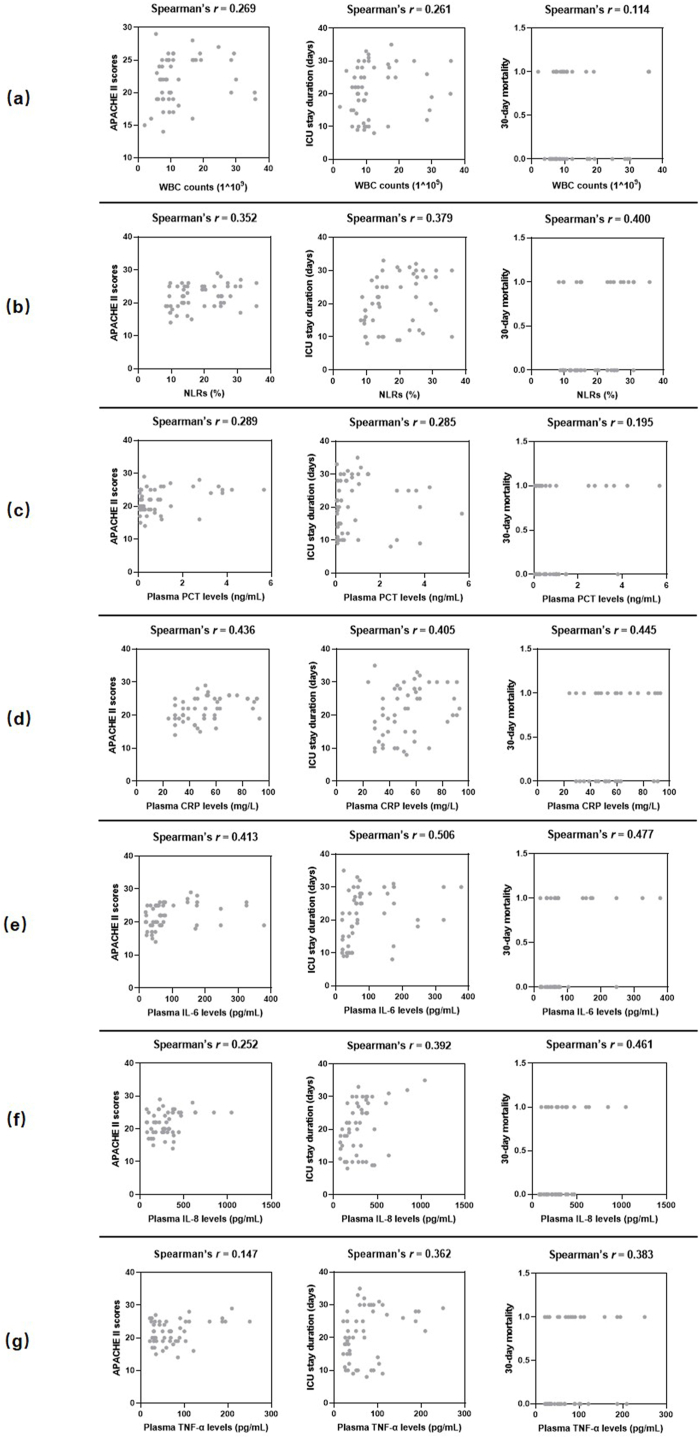
Scatterplot shows the correlations between inflammatory markers and disease severity. Inflammatory markers include WBC counts (a), NLRs (b), plasma PCT levels (c), plasma CRP levels (d), plasma IL-6 levels (e), plasma IL-8 levels (f), and plasma TNF-α levels (g). Disease severity was presented as APACHE II scores, ICU stay duration and 30-day mortality. r : Spearman's correlation conefficient. For the analysis, the ICU hospitalization days of patients who died within 30 days were also considered as part of the ICU stay duration.
In multivariate analyses, any confounding association between inflammatory markers and 30-day mortality were excluded using logistic regression analysis, considering patients' background variables, primarily age, number of major diseases, number of antibiotics used, and the foci of infection. Plasma CRP (OR = 1.130, 95% CI = 1.039–1.319, p = 0.027) and IL-6 (OR = 1.045, 95% CI = 1.017–1.100, p = 0.018) levels were independently associated with 30-day mortality ( Table 2 ).
Multivariate logistic regression analysis of factors for 30-day mortality.
CRP, C-reactive protein; IL-6, interleukin 6; IL-8, interleukin 8; n, number; NLR, neutrophil to lymphocyte ratio; PCT, procalcitonin; TNF-α, tumor necrosis factor alpha; WBC, white blood cell. p -value <0.05 means statistically significant.
4. Discussion
In the past decade, BSIs have been frequently seen in patients admitted to the ICU, in particular, with bacteremia and candidemia being linked to significant levels of morbidity and mortality [ 39 ]. Enterococcus species are a significant group of bacterial pathogens that lead to hospital-acquired BSIs in ICU patients, with E. faecalis and E . faecium being the most prevalent pathogens responsible for the majority of infections [ 40 ]. The prevalence of E . faecalis -induced BSIs is estimated to be around 4.5 infections per 100,000 individuals each year. Additionally, the case fatality rate linked to these infections ranges from 10% to 20% [ 41 ]. Besides bacterial BSIs, fungal infections, specifically those caused by Candida species, have demonstrated an increase, especially among individuals undergoing significant abdominal surgical procedures [ 42 ]. A previous study conducted by MacBrayne et al. [ 43 ] indicated that the median blood culture time to positivity in Gram-negative pathogens was 14.33 h, while in fungi it was 32.5 h, which aligns with the findings of current study. This study also identified that co-infection with Candida spp. prolonged the blood culture time to positivity of Enterococcus spp. Further, co-infections that involve both C . albicans and E . faecalis are on the rise in hospital-acquired BSIs [ 44 ]. Hospital-acquired BSIs are frequently linked to multidrug-resistant strains, which have a strong association with antibiotic usage in healthcare settings [ 45 ]. In the present study, it was found that BSIs caused by mixed cultures were the most prevalent, with a majority of these cases being hospital-acquired. These results indicate a potentially elevated risk of developing BSIs from mixed cultures within healthcare facilities. Among them, a significant number of patients received three or more antibiotics. In addition, Zeise et al. found that a considerable proportion of BSIs caused by E. faecalis and C. albicans were linked to central venous catheters [ 44 ]. Similarly, all patients with BSIs included in this study received central venous catheterization after ICU admission. BSI refers to a patient displaying signs of systemic infection with a positive blood culture. The infection may originate from a known source or an unidentified source [ 46 ]. Evans et al. [ 47 ] conducted a previous prospective cohort study on BSIs and reported that around 30% of patients had no identified foci of infection, which aligns with our findings. A prior prospective cohort study conducted in Taiwan [ 48 ] revealed that polymicrobial BSIs were linked to higher risk of poor outcomes in comparison to mono-microbial BSIs. Similarly, the present study also observed that patients with the mixed-pathogen BSIs exhibited a higher APACHE II score, longer ICU stay, and increased 30-day mortality compared to patients with the single-pathogen BSIs, further highlighting a significant increase in disease severity when multiple pathogens (both Candida spp. and Enterococcus spp.) at present. Inappropriate initial antimicrobial therapy is a crucial factor affecting the prognosis of patients with severe infections [ 35 ]. Several previous studies have highlighted that inappropriate initial antimicrobial therapy leads to an increase in both hospital mortality rate and length of stay among critically ill patients with Gram-negative BSIs [ 34 ]. In this study, it was observed that the proportion of patients in the mixed-pathogen BSI group who received inappropriate initial antimicrobial therapy was higher compared to the single-pathogen BSI group. However, it is important to note that this difference was not statistically significant. In a previous study conducted by Hamada et al. [ 49 ], it was found that prior use of antibiotics for uncertain foci was significantly associated with a 28-day mortality in patients with ampicillin-resistant enterococcal bacteremia. This factor may also contribute to the higher disease severity and poor prognosis in patients with mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. However, further studies with a larger sample size are needed to confirm these findings.
Systemic inflammation plays a crucial role in the advancement of diseases in BSIs and potentially sepsis, leading to heightened mortality risks [ 50 ]. Elevated NLRs are often characterized by increased neutrophils and decreased lymphocytes, reflecting an imbalance between acute inflammation and adaptive immunity [ 51 ]. A higher NLR, particularly >10, indicates a worse prognosis in patients with sepsis [ 23 ]. Salciccioli et al. found that higher NLRs in patients with sepsis were associated with higher disease severity characterized by a longer ICU stay and higher APACHE II score [ 52 ]. In the present study, the mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. resulted in higher NLRs than those of the single-pathogen BSIs by Enterococcus spp., and NLRs were also positively correlated with disease severity. The WBC counts, along with plasma PCT and CRP levels, are commonly used markers for identifying bacterial infection [ 53 ]. Yet, the predictive ability of WBC counts for BSIs is limited as a considerable number of individuals with BSI exhibit normal WBC counts [ 54 ]. Furthermore, studies have indicated that WBC counts are unable to distinguish between patients with fungal BSIs and those with negative blood cultures [ 55 ]. This explains why there were no significant differences in WBC counts between the two groups in the current study. Consistent with previous reports [ 56 , 57 ], WBC counts did not correlate with disease severity in ICU patients with BSIs, supporting that NLRs are a more valuable predictor of disease severity in the mixed-pathogen BSI caused by Enterococcus spp. and Candida spp.
PCT is recognized as a crucial marker of inflammation in clinical assessments of bacterial infections [ 58 ]. CRP is a valuable acute-phase protein that operates as a sensitive systemic marker for both inflammation and tissue injury [ 59 ]. Studies have shown a significant increase in plasma levels of both PCT and CRP in individuals with bacterial sepsis [ 58 ]. However, rare reports exist regarding the clinical significance of plasma PCT and CRP levels in cases of candidemia. Pieralli et al. found that critically ill patients in the ICU with sepsis and candidemia exhibited notably lower plasma PCT levels in comparison to those with bacteremia [ 60 ]. Li et al. [ 61 ] similarly reported that there was no significant difference in plasma PCT levels between patients with candidemia and bacteremia, but did find significantly higher plasma CRP levels in patients with candidemia. Our study also revealed that patients with mixed-pathogen BSIs involving Enterococcus spp. and Candida spp. had higher plasma CRP levels in comparison to PCT levels when compared to patients with single-pathogen BSIs.
In patients with bacteria-driven sepsis, the plasma PCT and CRP levels found to be linked to disease severity [ 62 ]. To determine if the same correlation applies to mixed-pathogen BSIs involving Enterococcus spp. and Candida spp., correlation analyses were conducted between inflammatory markers and disease severity. Contrary to previous findings [ 62 ], the present study found that plasma PCT levels did not correlate with disease severity in cases of mixed-BSIs caused by Enterococcus spp. and Candida spp. Interestingly, there was a positive correlation observed between plasma CRP levels and APACHE II scores, ICU stay duration, and 30-day mortality. This indicates that plasma CRP levels could serve as a more dependable marker for assessing disease severity and predicting outcomes in such infections. Several previous studies have consistently found that levels of PCT are higher in Gram-negative bacterial infections compared to Gram-positive bacterial and fungal infections. Additionally, PCT levels serve as a more sensitive marker for bacterial BSIs compared to fungal BSIs [ 63 ]. This discrepancy may elucidate the lack of a positive correlation between PCT levels and disease severity in the present study. Coexistence of C. albicans and bacteria like E. faecalis and Staphylococcus aureus has been shown to exacerbate bacterial infection, resulting in a heightened inflammatory response and ultimately a worse prognosis [ 64 ]. Based on our findings, we determined that higher disease severity in patients with mixed-pathogen BSIs was attributed to higher plasma CRP levels, which could be due to the exacerbation of bacterial infection in the presence of a fungal pathogen.
Pro-inflammatory cytokines IL-6, IL-8, and TNF-α are commonly activated during severe infections. In our previous study, plasma IL-6, IL-8, and TNF-α levels were observed to be higher during sepsis due to co-infections by Klebsiella pneumoniae and Acinetobacter baumannii in comparison to sepsis due to infection by only one of these species; further, the levels of these markers were correlated with disease severity [ 26 ]. Wang et al. [ 65 ] found that the levels of IL-6, IL-8, and TNF-α were significantly elevated in candidemia compared with Gram-positive bacteremia, indicating that the elevated levels of these pro-inflammatory cytokines may serve as valuable diagnostic markers for differentiating between candidemia and bacteremia. Co-infection by Candida albicans and Staphylococcus aureus has been proven to shift the balance between pro-inflammatory and anti-inflammatory cytokine production. Altered host responses may be due to direct host cell-microbe interactions, increased microbial transmission, or increased production of pro-inflammatory cytokines in host cell responses to co-infection [ 66 ]. In the present study, plasma IL-6, IL-8, and TNF-α levels in the mixed-pathogen BSI group were significantly higher than those in the single-pathogen BSI group, further supporting the previous findings. Additionally, we found that plasma IL-6, IL-8, and TNF-α levels were positively correlated with disease severity by Spearman's rank correlation analysis. These results indicated that IL-6 and CRP are of potential clinical significance in predicting the disease severity in critically ill patients with mixed-pathogen BSI caused by Enterococcus spp. and Candida spp. Furthermore, our findings again indicated that Candida spp. enhanced enterococcal infection and manifested as higher plasma levels of IL-6, IL-8, and TNF-α and associated disease severity.
While these findings indicated that NLRs, the plasma levels of CRP, IL-6, IL-8, and TNF-α were associated with disease severity, the strength of these associations was found to be weak. Additionally, it was noted that age, number of major diseases, number of antibiotics used, the foci of infection could not be disregarded when determining the impact on the mortality of patients with mixed-pathogen BSIs. Consequently, considering the aforementioned clinical context of the patients, we conducted a multivariate analysis taking multiple factors into account. The results revealed plasma CRP and IL-6 levels were independently associated with 30-day mortality, thereby reaffirming the significance of inflammatory markers in assessing disease severity.
This study had a few limitations. Firstly, it was conducted at a single-center ICU, which may limit the generalizability of the results. Secondly, patients with other bacterial co-infections, immunosuppression, and previous or ongoing corticosteroid treatments were excluded from the study, resulting in a further reduction in the sample size. Thirdly, despite the retrospective observational design of the study, the potential presence of confounding factors was not taken into account. Lastly, the study did not consider the potential impact of antibiotic prophylaxis based on full coverage of pathogenic microorganisms.
5. Conclusion
In summary, our study exposed that mixed-pathogen ( Enterococcus spp. and Candida spp.) BSIs in the ICU were predominantly hospital-acquired, with E. faecalis and C. albicans as the main pathogens. The majority of this patient cohort consisted mainly of elderly individuals with cardiovascular disease, neurological disease, diabetes, and other underlying conditions. Moreover, the patients underwent multiple antimicrobial therapies, including an initial antimicrobial therapy that may have been inappropriate to some extent. Additionally, they received invasive interventions such as central venous catheterization and urethral catheterization as part of their necessary treatment. By analyzing our findings, it was observed that patients with mixed-pathogen BSIs exhibited higher NLRs and plasma CRP, IL-6, IL-8, and TNF-α levels as well as higher disease severity when compared to those with single-pathogen ( Enterococcus spp.) BSIs. This result could be due to the enhancement of enterococcal infection by Candida spp.; however, further studies are required to confirm this effect. Additionally, this study revealed that NLRs as well as plasma CRP, IL-6, IL-8, and TNF-α levels were positively correlated with disease severity in patients with the mixed-pathogen BSIs caused by both Enterococcus spp. and Candida spp. Among these markers, plasma CRP and IL-6 levels exhibited independent associations with 30-day mortality. In addition, multivariate logistic regression analysis revealed that there were correlations between NLRs and plasma IL-8 levels, as well as age and the number of major diseases, with 30-day mortality. However, these correlations were not statistically significant, possibly due to limitations in sample size. Hence, these inflammatory markers may be of great clinical significance as predictors of the disease severity in this specific patient population. Furthermore, our study may provide insights for future larger multicenter prospective studies.
Funding statement
This work was supported by National Major Science and Technology Projects of China (No. 2018ZX10713-002-002-004). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability statement
Data will be made available on request.
Ethical statement
This study was approved by the Medical Ethics Committee of The Fifth Medical Center of Chinese PLA General Hospital [ky-2020-8-18]. Informed consent was not required as the study was retrospective in nature, and all data sources were anonymized.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Xin Wang: Writing – original draft, Investigation, Data curation. Ming Li: Writing – original draft, Data curation. Yang Yang: Writing – original draft, Software, Data curation. Xueyi Shang: Software, Data curation. Yonggang Wang: Writing – review & editing, Conceptualization. Yan Li: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge Editideas ( www.editideas.cn ) for English language editing.
Contributor Information
Xin Wang, Email: [email protected].
Ming Li, Email: [email protected].
Yang Yang, Email: [email protected].
Xueyi Shang, Email: [email protected].
Yonggang Wang, Email: [email protected].
Yan Li, Email: [email protected].
Abbreviations
Acute Physiology and Chronic Health Evaluation
bloodstream infection
confidence interval
C-reactive protein
hospital information system
intensive care unit
interleukin-6
interleukin-8
neutrophil-to-lymphocyte ratios
procalcitonin
tumor necrosis factor-α;
white blood count
- 1. Ike Y. [Pathogenicity of enterococci] Nihon Saikingaku Zasshi. 2017;72(2):189–211. doi: 10.3412/jsb.72.189. Japanese. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Fiore E., Van Tyne D., Gilmore M.S. Pathogenicity of enterococci. Microbiol. Spectr. 2019;7(4) doi: 10.1128/microbiolspec.GPP3-0053-2018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Kang J., Sickbert-Bennett E.E., Brown V.M., Weber D.J., Rutala W.A. Relative frequency of health care-associated pathogens by infection site at a university hospital from 1980 to 2008. Am. J. Infect. Control. 2012;40(5):416–420. doi: 10.1016/j.ajic.2011.06.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. García-Solache M., Rice L.B. The Enterococcus: a model of adaptability to its environment. Clin. Microbiol. Rev. 2019;32(2) doi: 10.1128/CMR.00058-18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Sava I.G., Heikens E., Huebner J. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 2010;16(6):533–540. doi: 10.1111/j.1469-0691.2010.03213.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Raza T., Ullah S.R., Mehmood K., Andleeb S. Vancomycin resistant Enterococci: a brief review. J. Pakistan Med. Assoc. 2018;68(5):768–772. [ PubMed ] [ Google Scholar ]
- 7. Köhler J.R., Casadevall A., Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med. 2014;5(1):a019273. doi: 10.1101/cshperspect.a019273. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. von Lilienfeld-Toal M., Wagener J., Einsele H., Cornely O.A., Kurzai O. Invasive fungal infection. Dtsch Arztebl Int. 2019;116(16):271–278. doi: 10.3238/arztebl.2019.0271. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Frange P., Bougnoux M.E., Lanternier F., Neven B., Moshous D., Angebault C., et al. An update on pediatric invasive aspergillosis. Med. Maladies Infect. 2015;45(6):189–198. doi: 10.1016/j.medmal.2015.04.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Pathakumari B., Liang G., Liu W. Immune defence to invasive fungal infections: a comprehensive review. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110550. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Huerta L.E., Rice T.W. Pathologic difference between sepsis and bloodstream infections. J Appl Lab Med. 2019;3(4):654–663. doi: 10.1373/jalm.2018.026245. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Guna Serrano M.R., Larrosa Escartín N., Marín Arriaza M., Rodríguez Díaz J.C. Microbiological diagnosis of bacteraemia and fungaemia: blood cultures and molecular methods. Enferm. Infecc. Microbiol. Clín. 2019;37(5):335–340. doi: 10.1016/j.eimc.2018.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Fu Y., Wang W., Zeng Q., Wang T., Qian W. Antibiofilm efficacy of luteolin against single and dual species of Candida albicans and Enterococcus faecalis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.715156. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Rao Y., Shang W., Yang Y., Zhou R., Rao X. Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front. Microbiol. 2020;11:1000. doi: 10.3389/fmicb.2020.01000. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Theilacker C., Sanchez-Carballo P., Toma I., Fabretti F., Sava I., Kropec A., et al. Glycolipids are involved in biofilm accumulation and prolonged bacteraemia in Enterococcus faecalis. Mol. Microbiol. 2009;71(4):1055–1069. doi: 10.1111/j.1365-2958.2008.06587.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Epelbaum O., Chasan R. Candidemia in the intensive care unit. Clin. Chest Med. 2017;38(3):493–509. doi: 10.1016/j.ccm.2017.04.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Golli A.L., Cristea O.M., Zlatian O., et al. Prevalence of multidrug-resistant pathogens causing bloodstream infections in an intensive care unit. Infect. Drug Resist. 2022;15:5981–5992. doi: 10.2147/IDR.S383285. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Magill S.S., Edwards J.R., Bamberg W., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Wu Y.M., Huang P.Y., Cheng Y.C., Lee C.H., Hsu M.C., Lu J.J., et al. Enhanced virulence of Candida albicans by Staphylococcus aureus: evidence in clinical bloodstream infections and infected zebrafish embryos. J Fungi (Basel). 2021;7(12):1099. doi: 10.3390/jof7121099. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Molano Franco D., Arevalo-Rodriguez I., Roqué I Figuls M., Montero Oleas N.G., Nuvials X., Zamora J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst. Rev. 2019;4(4):CD011811. doi: 10.1002/14651858.CD011811.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Huerta L.E., Rice T.W. Pathologic difference between sepsis and bloodstream infections. J Appl Lab Med. 2019;3(4):654–663. doi: 10.1373/jalm.2018.026245. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Abe M., Kinjo Y., Ueno K., et al. Differences in ocular complications between Candida albicans and non-albicans Candida infection analyzed by epidemiology and a mouse ocular candidiasis model. Front. Microbiol. 2018;9:2477. doi: 10.3389/fmicb.2018.02477. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Drăgoescu A.N., Pădureanu V., Stănculescu A.D., Chiuțu L.C., Tomescu P., Geormăneanu C., et al. Neutrophil to lymphocyte ratio (NLR)-A useful tool for the prognosis of sepsis in the ICU. Biomedicines. 2021;10(1):75. doi: 10.3390/biomedicines10010075. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Cortegiani A., Misseri G., Ippolito M., et al. Procalcitonin levels in candidemia versus bacteremia: a systematic review. Crit. Care. 2019;23(1):190. doi: 10.1186/s13054-019-2481-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Xiao L., Ran X., Zhong Y., Le Y., Li S. A combined ratio change of inflammatory biomarkers at 72 h could predict the severity and prognosis of sepsis from pulmonary infections. Immunobiology. 2022;227(6) doi: 10.1016/j.imbio.2022.152290. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Wang X., Zhang Q., Yan Y., Yang Y., Shang X., Li Y. Clinical significance of pro-inflammatory cytokines and their correlation with disease severity and blood coagulation in septic patients with bacterial Co-infection. Shock. 2021;56(3):396–402. doi: 10.1097/SHK.0000000000001735. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Dongari-Bagtzoglou A., Wen K., Lamster I.B. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 1999;14(6):364–370. doi: 10.1034/j.1399-302x.1999.140606.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Taj-Aldeen S.J., Mir F.A., Sivaraman S.K., AbdulWahab A. Serum cytokine profile in patients with candidemia versus bacteremia. Pathogens. 2021;10(10):1349. doi: 10.3390/pathogens10101349. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Chang Y.C., Chen J.S., Yin C.H., Shin-Jung Lee S., Chen W.C. Candidemia in hospitalized cirrhotic patients with bloodstream infection: a retrospective analysis and brief summary of published studies. J. Chin. Med. Assoc. 2022;85(3):295–303. doi: 10.1097/JCMA.0000000000000695. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Xiao H., Jia H., Yuan X., Zhou Q., Li W., Shan H., et al. The value of dynamic monitoring of procalcitonin in the early identification of pathogens and prognosis of bloodstream infections in the ICU. Ann. Palliat. Med. 2021;10(12):12208–12217. doi: 10.21037/apm-21-3232. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Song H., Liu J., Cao Z., Luo W., Chen J.Y. Analysis of disease profile, and medical burden by lead exposure from hospital information systems in China. BMC Publ. Health. 2019;19(1):1170. doi: 10.1186/s12889-019-7515-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Cuijpers M.L., Vos F.J., Bleeker-Rovers C.P., Krabbe P.F., Pickkers P., van Dijk A.P., et al. Complicating infectious foci in patients with Staphylococcus aureus or Streptococcus species bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26(2):105–113. doi: 10.1007/s10096-006-0238-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Larsen I.K., Pedersen G., Schønheyder H.C. Bacteraemia with an unknown focus: is the focus de facto absent or merely unreported? A one-year hospital-based cohort study. APMIS. 2011;119(4–5):275–279. doi: 10.1111/j.1600-0463.2011.02727.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Zilberberg M.D., Shorr A.F., Micek S.T., Vazquez-Guillamet C., Kollef M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit. Care. 2014;18(6):596. doi: 10.1186/s13054-014-0596-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Tang Y., Wu X., Cheng Q., Li X. Inappropriate initial antimicrobial therapy for hematological malignancies patients with Gram-negative bloodstream infections. Infection. 2020;48(1):109–116. doi: 10.1007/s15010-019-01370-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Sengupta M., Sarkar S., SenGupta M., Ghosh S., Sarkar R., Banerjee P. Biofilm producing Enterococcus isolates from vaginal microbiota. Antibiotics (Basel). 2021;10(9):1082. doi: 10.3390/antibiotics10091082. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Hii I.M., Liu C.E., Lee Y.L., Liu W.L., Wu P.F., Hsieh M.H., et al. Resistance rates of non-albicans Candida infections in taiwan after the revision of 2012 clinical and laboratory standards Institute breakpoints. Infect. Drug Resist. 2019;12:235–240. doi: 10.2147/IDR.S184884. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem. Med. 2016;26(1):5–16. doi: 10.11613/BM.2016.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Laupland K.B. Incidence of bloodstream infection: a review of population-based studies. Clin. Microbiol. Infect. 2013;19(6):492–500. doi: 10.1111/1469-0691.12144. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33(3) doi: 10.1128/CMR.00181-19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Billington E.O., Phang S.H., Gregson D.B., Pitout J.D., Ross T., Church D.L., Laupland K.B., Parkins M.D. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int. J. Infect. Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Pongrácz J., Kristóf K. Candida bloodstream infection: a clinical microbiology laboratory perspective. Acta Microbiol. Immunol. Hung. 2014;61(3):389–398. doi: 10.1556/AMicr.61.2014.3.11. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. MacBrayne C.E., Williams M.C., Prinzi A., Pearce K., Lamb D., Parker S.K. Time to blood culture positivity by pathogen and primary service. Hosp. Pediatr. 2021;11(9):953–961. doi: 10.1542/hpeds.2021-005873. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Zeise K.D., Woods R.J., Huffnagle G.B. Interplay between Candida albicans and lactic acid bacteria in the gastrointestinal tract: impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity. Clin. Microbiol. Rev. 2021;34(4) doi: 10.1128/CMR.00323-20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence. 2016;7(3):252–266. doi: 10.1080/21505594.2016.1159366. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Timsit J.F., Ruppé E., Barbier F., Tabah A., Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi: 10.1007/s00134-020-05950-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Evans R.N., Pike K., Rogers C.A., Reynolds R., Stoddart M., Howe R., et al. Modifiable healthcare factors affecting 28-day survival in bloodstream infection: a prospective cohort study. BMC Infect. Dis. 2020;20(1):545. doi: 10.1186/s12879-020-05262-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Yo C.H., Hsein Y.C., Wu Y.L., Hsu W.T., Ma M.H., Tsai C.H., et al. Clinical predictors and outcome impact of community-onset polymicrobial bloodstream infection. Int. J. Antimicrob. Agents. 2019;54(6):716–722. doi: 10.1016/j.ijantimicag.2019.09.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Hamada Y., Magarifuchi H., Oho M., Kusaba K., Nagasawa Z., Fukuoka M., et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J. Infect. Chemother. 2015;21(7):527–530. doi: 10.1016/j.jiac.2015.04.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Zheng C.F., Liu W.Y., Zeng F.F., Zheng M.H., Shi H.Y., Zhou Y., et al. Prognostic value of platelet-to-lymphocyte ratios among critically ill patients with acute kidney injury. Crit. Care. 2017;21(1):238. doi: 10.1186/s13054-017-1821-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Buonacera A., Stancanelli B., Colaci M., Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int. J. Mol. Sci. 2022;23(7):3636. doi: 10.3390/ijms23073636. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Salciccioli J.D., Marshall D.C., Pimentel M.A., et al. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit. Care. 2015;19(1):13. doi: 10.1186/s13054-014-0731-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Crouser E.D., Parrillo J.E., Seymour C., Angus D.C., Bicking K., Tejidor L., et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152(3):518–526. doi: 10.1016/j.chest.2017.05.039. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Marik P.E., Stephenson E. The ability of Procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database. J. Crit. Care. 2020;60:135–139. doi: 10.1016/j.jcrc.2020.07.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Wang Q., Yang M., Wang C., Cui J., Li X., Wang C. Diagnostic efficacy of serum cytokines and chemokines in fungal bloodstream infection in febrile patients. J. Clin. Lab. Anal. 2020;34(4) doi: 10.1002/jcla.23149. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Murray C.K., Hoffmaster R.M., Schmit D.R., Hospenthal D.R., Ward J.A., Cancio L.C., et al. Evaluation of white blood cell count, neutrophil percentage, and elevated temperature as predictors of bloodstream infection in burn patients. Arch. Surg. 2007;142(7):639–642. doi: 10.1001/archsurg.142.7.639. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Djordjevic D., Rondovic G., Surbatovic M., Stanojevic I., Udovicic I., Andjelic T., et al. Neutrophil-to-Lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/3758068. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Hu L., Shi Q., Shi M., Liu R., Wang C. Diagnostic value of PCT and CRP for detecting serious bacterial infections in patients with fever of unknown origin: a systematic review and meta-analysis. Appl. Immunohistochem. Mol. Morphol. 2017;25(8):e61–e69. doi: 10.1097/PAI.0000000000000552. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Simon L., Gauvin F., Amre D.K., Saint-Louis P., Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 2004;39(2):206–217. doi: 10.1086/421997. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Perrella A., Giuliani A., De Palma M., Castriconi M., Molino C., Vennarecci G., et al. C-reactive protein but not procalcitonin may predict antibiotic response and outcome in infections following major abdominal surgery. Updates Surg. 2022;74(2):765–771. doi: 10.1007/s13304-021-01172-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Li C., Cao J., Wang L., Jia X., He J., Zhang L. Up-regulation of chemokine CXCL13 in systemic candidiasis. Clin. Immunol. 2018;191:1–9. doi: 10.1016/j.clim.2017.11.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Zhao L., Zang X., Chen W., Sheng B., Gu X., Zhang J. [Analysis of correlation between inflammatory parameters and severity of sepsis caused by bacterial bloodstream infection in septic patients] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(6):448–453. doi: 10.3760/cma.j.issn.2095-4352.2015.06.007. Chinese. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Wu H.N., Yuan E.Y., Li W.B., Peng M., Zhang Q.Y., Xie K.L. Microbiological and clinical characteristics of bloodstream infections in general intensive care unit: a retrospective study. Front. Med. 2022;9 doi: 10.3389/fmed.2022.876207. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Garsin D.A., Lorenz M.C. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microb. 2013;4(5):409–415. doi: 10.4161/gmic.26040. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Wang Q., Wang C., Yang M., Li X., Cui J., Wang C. Diagnostic efficacy of serum cytokines and chemokines in patients with candidemia and bacteremia. Cytokine. 2020;130 doi: 10.1016/j.cyto.2020.155081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Allison D.L., Scheres N., Willems H.M.E., Bode C.S., Krom B.P., Shirtliff M.E. The host immune system facilitates disseminated Staphylococcus aureus disease due to phagocytic attraction to Candida albicans during coinfection: a case of bait and switch. Infect. Immun. 2019;87(11) doi: 10.1128/IAI.00137-19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (3.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
Inflammatory markers dominant in the literature are acute phase response (APR)-associated and include fibrinogen, C-reactive protein (CRP) and, more recently, interleukin- (IL-) 6. This thesis reviews the literature and suggests the need for further research into novel inflammatory markers of CVD risk.
These inflammatory biomarkers respond to the psychosocial stressors associated with depression, and these both precede and follow diagnosis with major depressive disorder (Miller & Raison, 2016). Inflammatory biomarkers, such as adiponectin, hs-CRP, leptin, IL1-β, IL6, and TNFα affect the effectiveness of newly developed antidepressants.
Oxidative and Inflammatory Markers within Advanced Atherosclerotic Plaques A thesis submitted in partial fulfilment of the Requirements for the degree of Master of Science in Biochemistry At the School of Biological Sciences University of Canterbury New Zealand Elizabeth M. Crone 2008
Research has demonstrated that inflammation is a central part of several psychiatric disorders, of which depression in the most researched condition. It has been suggested that patterns and levels of systemic inflammatory markers could be used as gauging tools in the diagnosis and assessment of psychiatric disorders. Most studies have, however ...
Despite calprotectin’s high sensitivity and specificity, it ought not to be considered as a specific marker of inflammation, since increased levels are also found in neoplasia, polyps, microscopic colitis, allergic colitis, active celiac disease, infections, nonsteroidal anti-inflammatory enteropathy, increasing age, etc. [1, 6].
EVALUATION OF BONE BIOCHEMICAL MARKERS AND INFLAMMATORY MARKERS IN YEARLINGS FED VARYING RATIOS OF OMEGA-6 AND OMEGA-3 POLYUNSATURATED FATTY ACIDS A Thesis by TRINETTE NOEL ROSS Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2006
We selected inflammation markers that showed adequate temporal reproducibility (ICC ≥ 0.5) over a 2–3 year period in preliminary reproducibility studies, thus suggesting that a single inflammation marker measurement can be used to rank women according to their average level [18–20]. 2. Materials and Methods 2.1 Study Subjects
The aim of our study was to compare concentrations of inflammatory markers in patients with PTSD compared with healthy controls. Methods: We did a meta-analysis and meta-regression of studies comparing inflammatory markers between patients with PTSD and healthy controls by searching PubMed, Embase, Scopus, Web of Science, and PsycINFO for ...
To date, however, inflammatory markers are generally measured in venous blood . Repeatedly collecting venous blood in an everyday environment would be far too invasive and unpractical. Fortunately, some inflammatory markers are also excreted in urine and saliva [15,16], which can be obtained in a non
Mar 3, 2024 · Based on the aforementioned research, we proposed that inflammatory markers, including white blood cell (WBC) counts, NLRs, and plasma PCT, CRP, IL-6, IL-8, and TNF-α levels might be of clinical significance in assessing the disease severity in critically ill patients with mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp.