Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts

Articles in 2024
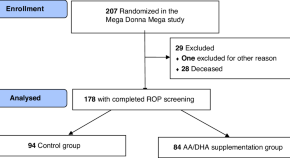
Platelet characteristics in extremely preterm infants after fatty acid supplementation: a randomized controlled trial
- Pia Lundgren
- Aldina Pivodic
- Ann Hellström
Genetic advances in sporadic tetralogy of Fallot: discovery of novel HAND1 promoter variants
- Jamie Gilley
- Binoy Shivanna
Body composition in prepubertal children with idiopathic premature adrenarche: implications for cardiometabolic health
- Asaf Ben Simon
- Michal Yackobovitch-Gavan
- Yael Lebenthal
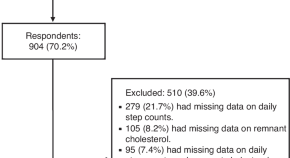
Daily steps, cardiorespiratory fitness, and remnant cholesterol in schoolchildren: mediation effects for cardiovascular prevention
- Eva Rodríguez-Gutiérrez
- Vicente Martínez-Vizcaíno
- Ana Torres-Costoso
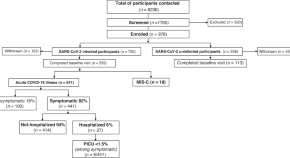
Pediatric SARS-CoV-2 long term outcomes study (PECOS): cross sectional analysis at baseline
- Gina A. Montealegre Sanchez
- Lauren E. Arrigoni
- Roberta L. DeBiasi
High speed, high risk: the rise of e-scooter injuries in adolescents
- Katherine E. Douglas
- Andrew M. Fine
Prioritizing caregiver mental health to promote child health
- Kristine Schmitz
- Christina R. Rojas
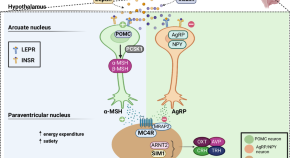
The expanding landscape of genetic causes of obesity
- Ekaterina Semenova
- Vidhu V. Thaker
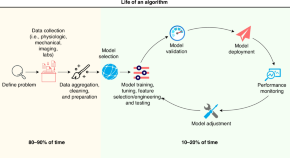
AI models in clinical neonatology: a review of modeling approaches and a consensus proposal for standardized reporting of model performance
- Ameena Husain
- Lindsey Knake
- Zachary Vesoulis
Can we prevent extrauterine growth restriction by doing metabolic analysis?
- Fuat Emre CANPOLAT

Correction: Patent Ductus Arteriosus: (Don’t) follow your heart
- Rahul Mital
- Martin L. Bocks
- Timothy J. Mead
Recent challenges in children’s developmental milestones
- Shirin Shamel
- Mohammad Reza Zarkesh

Comparing machine learning techniques for neonatal mortality prediction: insights from a modeling competition
- Brynne A. Sullivan
- Alvaro G. Moreira
- Zachary A. Vesoulis
Extrarenal manifestations of atypical hemolytic uremic syndrome: a systematic review and meta-analysis
- Abdel Yusuf
- Rupesh Raina
Correction: Science for kids: skin color influences transcutaneous bilirubin measurements: a systematic in vitro evaluation
- Ritika Chalise
- Capri Faranetta
- Sheila Gilligan
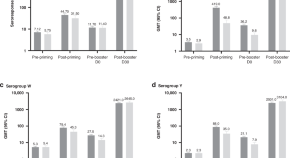
Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in children aged 10–12 years: phase III randomized trial complementary analysis of immune persistence 3–6 years after priming
- James Peterson
- Katherine Galarza
- Betzana Zambrano
The importance of investigating the effects of drug therapy on maternal breast milk composition
- Jill L. Maron
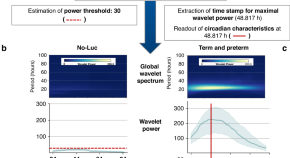
Circadian clock activity in human umbilical vein endothelial cells of preterm and term neonates
- Achim Kramer
- Christof Dame
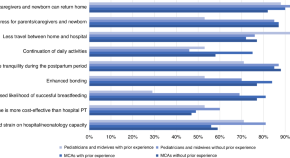
Home phototherapy for neonatal hyperbilirubinemia: current practices and attitudes
- Maryse C. Cnossen
- Jessie Spaan
- Hanneke W. Harmsen van der Vliet – Torij
Oxygen affinity of fetal and adult hemoglobin in action: shifting our understanding of red blood cell transfusions in neonates
- Nina A. M. Houben
- Enrico Lopriore
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES