
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- What is the weather like in Paris?
- What is the landscape of Paris?
- Is mathematics a physical science?
- Why does physics work in SI units?


Charles-Augustin de Coulomb
Our editors will review what you’ve submitted and determine whether to revise the article.
Charles-Augustin de Coulomb (born June 14, 1736, Angoulême , France—died August 23, 1806, Paris) was a French physicist best known for the formulation of Coulomb’s law , which states that the force between two electrical charges is proportional to the product of the charges and inversely proportional to the square of the distance between them. The Coulomb force is one of the principal forces involved in atomic reactions.
Coulomb spent eight years, from 1764 to 1772, at Martinique in the West Indies as a military engineer and returned to France with impaired health, from which he suffered for the rest of his life. Upon the outbreak of the French Revolution , he retired to a small estate at Blois and devoted himself to scientific research. In 1802 he was appointed an inspector of public instruction.

Coulomb developed his law as an outgrowth of his attempt to investigate the law of electrical repulsions as stated by Joseph Priestley of England. To this end he invented sensitive apparatus including a torsion balance to measure the electrical forces involved in Priestley’s law and published his findings in 1785–89. He also established the inverse square law of attraction and repulsion of unlike and like magnetic poles, which became the basis for the mathematical theory of magnetic forces developed by Siméon-Denis Poisson . He also did research on friction of machinery, on windmills , and on the elasticity of metal and silk fibres. The coulomb , a unit of electric charge, was named in his honour.
The National MagLab is funded by the National Science Foundation and the State of Florida.
Torsion Balance – 1785
Charles-Augustin de Coulomb didn't invent the torsion balance, but he was the first to discover it could be used to measure electrical charge – the first device capable of such a feat.

In early investigations of electricity, scientists had few tools to aid them. By the 1780s, devices to generate, store and detect static electricity had been built, but there was no easy way to gauge amounts of static electrical charges. A French engineer with an interest in electricity and magnetism, Charles-Augustin de Coulomb , developed one of the earliest instruments capable of this feat: the torsion balance.
Coulomb’s torsion balance consists of several small parts, pictured at right. Within a glass case, which prevents breezes or other environmental factors from affecting results, a needle hangs from a thread, typically of silk. A narrow glass tube extends through the top of the glass case. At the top of the tube is a metal sphere, from which the needle hangs by the thread. A small metal sphere is at one end of the needle, which can swing freely due to its suspended state. Protruding through the top of the glass case is also a metal rod with metal spheres at both ends (one inside the case, one outside).
To use the torsion balance, Coulomb would hold an object near the metal sphere at the upper end of the metal rod. Any charge held by the object being studied would transfer to the metal sphere, then travel along the rod to the sphere at the other end. There the charge could affect the needle suspended in the case, which in its resting state touched the rod's lower sphere. So any charge in that sphere passed to the needle’s sphere. Once the rod’s sphere and the needle’s sphere both became similarly charged, they repelled one another. The repulsion caused the needle to move and the thread holding it to twist. The twisting action is called torsion, hence the name of the instrument. To determine how much torsion occurred, Coulomb consulted a small scale marked in degrees near the upper end of the narrow glass tube. A second scale encircling the glass case itself allowed him to determine how far the needle moved. As Coulomb realized, the greater the charge, the greater the torsion and displacement he observed.
An earlier scientist, John Michell, had used a similar instrument to study gravity, but the device did not gain much fame until after Coulomb reinvented it and put it to a different use. Coulomb carried out detailed studies of electrostatic forces with the torsion balance that allowed him to offer the world proof of the inverse square law that today bears his name. According to Coulomb’s law , the electric force between objects is inversely proportional to the distance between the objects. A similar inverse square law exists for gravity, but gravitation is influenced by the masses of objects rather than their charges.
After Coulomb published the results of his investigations and a description of the torsion balance, scientists around the globe wanted the tool. In fact, the torsion balance became one of the most popular scientific instruments to grace laboratories in the late 18th century and throughout the following century.
More Stories

Oscilloscope – 1897
Iconoscope – 1923

Planté Battery – 1859
Charles de Coulomb

(1736-1806)
Who Was Charles de Coulomb?
Charles-Augustin de Coulomb studied engineering and plied his trade with the military before winning accolades for his work in torsion balances. He offered pioneering theories in the force found between electrical charges, as well as magnetic attraction and repulsion. The unit of measurement known as the coulomb is named in his honor. He died in Paris on August 23, 1806.
Charles-Augustin de Coulomb was born in Angoulême, France, on June 14, 1736, and went on to become one of the most important scientists in the early discovery of electricity. Both of his parents, Henri Coulomb, a lawyer, and Catherine Bajet, came from well-established aristocratic families in Angoulême, France. Soon, his family moved to Paris, where he studied mathematics and attended the Collège des Quatre-Nations.
Military Career
Coulomb enrolled in military school in 1759, graduating from the Royal Engineering School of Mézières (École royale du génie de Mézières) in 1761. Early in his career, Coulomb worked in structural design and soil mechanics. Over the next 20 years, he was stationed in a number of locations. Beginning in 1764, he served nine years in Martinique, West Indies, and was in charge of building Fort Bourbon.
After falling ill with fever, in 1773, Coulomb returned to France and began some of his most important work on applied mechanics. That same year, he presented his first scholarly paper, "Statistical Problems applied to Architecture," to the Académie of Sciences. His use of calculus to overcome various discrepancies in engineering issues highly impressed the Académie.
In 1779, Coulomb was sent to Rochefort, France, to supervise the construction of a fort made entirely of wood. During this time, Coulomb used the shipyards at Rochefort for his research on friction and the stiffness of ropes. These experiments led to his major work, Theorie des Machines Simples ("Theory of Simple Machines"), in 1781, which won him the Grand Prix of the Académie of Sciences.
Controversy and Absolution
That same year, Coulomb was appointed to report on the feasibility of a navigable canal in Brittany. He condemned the plan as expensive and unprofitable, but the French bureaucracy saw it differently and, thusly, temporarily penalized him. Indignant, Coulomb resigned, but was rejected. When asked to reevaluate the project, he came up with the same conclusions. An independent examination proved that he was right and he was rewarded for his efforts, but the experience soured him, and from this point, on he devoted his time to the study of physics.
In 1784, Coulomb published a paper on the elasticity of wires under twisting stress. This led to his well-known study of torsion balance, which was subsequently used to determine the density of the earth. But most effectively, the process was used as a way of measuring the forces of frictional electricity and magnetism by de Coulomb himself.
Between 1785 and 1791, Coulomb wrote seven crucial papers that dealt with various aspects of electricity and magnetism. This led him to formulate the theory known as Coulomb's Law, which verified that the force between two electrical charges is proportional to the product of the charges and inversely proportional to the square of the distance between them.
Later Years and Death
When the French Revolution began, Coulomb, like many aristocrats, was expelled from government. In 1791, he retired from the Corps du Genie and lived on his estate at Blois, deeply involved in scientific research. During this time, he investigated the friction of pivots, viscosity of fluids and energy of men affected by food and climate.
Coulomb's second son was born on July 30, 1797, and in 1802, the physicist married the mother of his two sons, Louise Francoise LeProust Desormeaux. Since his service in the West Indies, de Coulomb had suffered from chronic ailments. He fell ill with a slow fever in the summer of 1796, and died in Paris on August 23, 1806.
QUICK FACTS
- Name: Charles-Augustin de Coulomb
- Birth Year: 1736
- Birth date: June 14, 1736
- Birth City: Angoulême
- Birth Country: France
- Gender: Male
- Best Known For: French engineer and physicist Charles de Coulomb made pioneering discoveries in electricity and magnetism, and came up with the theory called Coulomb's Law.
- War and Militaries
- Politics and Government
- Science and Medicine
- Astrological Sign: Gemini
- Royal Engineering School of Mézières (École royale du génie de Mézières)
- Collège des Quatre-Nations
- Nacionalities
- Death Year: 1806
- Death date: August 23, 1806
- Death City: Paris
- Death Country: France
CITATION INFORMATION
- Article Title: Charles de Coulomb Biography
- Author: Biography.com Editors
- Website Name: The Biography.com website
- Url: https://www.biography.com/scientists/charles-de-coulomb
- Access Date:
- Publisher: A&E; Television Networks
- Last Updated: May 17, 2021
- Original Published Date: April 2, 2014
- The sciences are monuments devoted to the public good; each citizen owes to them a tribute proportional to his talents.
Watch Next .css-16toot1:after{background-color:#262626;color:#fff;margin-left:1.8rem;margin-top:1.25rem;width:1.5rem;height:0.063rem;content:'';display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;}

Famous Scientists

Stephen Hawking
Chien-Shiung Wu

The Solar Eclipse That Made Albert Einstein a Star

Jane Goodall

Marie Curie

Howard Carter, King Tut's Tomb, and a Deadly Curse

Benjamin Banneker

Neil deGrasse Tyson

Daniel Hale Williams

Patricia Bath

Mae Jemison
June 1785: Coulomb Measures the Electric Force

Around 600 BC, the Greek philosopher Thales wrote that when he rubbed pieces of amber with fur, the amber attracted bits of straw and other small objects. When scientists began to study the phenomenon, they already had a word for it, thanks to Thales: "electricity,” derived from “elektron,” the Greek word for amber. In studying this force, others observed that charged objects sometimes attract one another and sometimes repel. Twenty-three centuries later, Benjamin Franklin attributed this effect to the existence of two electrical fluids, one positive and the other negative.
Much of the modern physical description of electrical forces comes from careful experiments done by the French scientist Charles Augustin Coulomb (1736-1806). His parents came from wealthy families living near Montpellier 1 , and they moved to Paris when Coulomb’s father began work there. Coulomb earned a degree at the engineering school at Mezieres and became a lieutenant in the military engineering force.
As a trained army engineer, he received several assignments in France. In 1764, Coulomb went to Martinique to supervise the construction of a fort. Coulomb oversaw the construction from 1764 to 1772, and then he returned to France. His health, impaired by the tropical ailments of Martinique, would trouble him for the rest of his life. With his return, his attention also shifted — after many projects in engineering, he began to work on pure physics.
Coulomb became interested in measuring the electrical force between small charged objects and perfected a torsion balance which could reliably measure such small forces 2 . He suspended a needle on a fine fiber of silver, copper, or silk. The needle held a small electrically charged pith ball at one end and a counterweight at the other end, balanced so that the needle could rotate in a horizontal plane. The calibrated torsion balance measured the force needed to twist the needle through a given angle.
By bringing a similarly charged pith ball near the one on the needle, Coulomb determined the repulsive force between the charged balls as a function of their separation. With these experiments, he launched the quantitative study of electric force. He wrote “The repulsive force of two small globes with the same nature of electricity is inversely proportional to the square of the distance between the centers of the two globes” 3 .
When the two pith balls had charges of opposite sign, the experiment described above did not work well. If the balls came too close to one another, they would jump together and stick, ending the experiment. With difficulty, he did measure the relation between force and separation in this case, but he decided to use a completely independent method to confirm the result [ 3 ]. He suspended a needle with a small plate on one end, and the plate was then charged. The opposite charge was placed on the surface of a hollow sphere of copper or metal-coated cardboard, about a foot in diameter.
Coulomb assumed that the large sphere would behave as if all its charge were concentrated in a point at its center. The needle was made to oscillate in a narrow arc in the horizontal plane. The period of oscillation depended on the force between the charged sphere and the charged plate on the needle, just as the period of the ordinary simple pendulum depends on the force exerted by gravity. Coulomb then measured the period of oscillation at various distances from the large sphere and, using an equation similar to that for the pendulum, related the period to the force between the charges.
The result: Coulomb’s law 4 . “We have arrived here by a method absolutely different from the first ... to conclude that the attraction of the electric fluid called ‘positive’ for the electric fluid, ordinarily called ‘negative,’ is as the inverse square of the distance.” He went on to show that, for a charged metal object or other conducting object, all the charge resides on the surface, no matter the shape of the object 5 .
Coulomb’s law underlies much of atomic physics. The attractive force F between an electron of charge e a distance r from a nucleus of atomic number Z and charge Ze is F = Ze2/r2 . Ernest Rutherford, studying the scattering of alpha particles, used this equation to show that the diameter of the atomic nucleus is orders of magnitude less than that of the atom — i.e., that the nucleus is effectively a point mass. Later, Niels Bohr used this result as the starting point of his theory of the line spectrum of the hydrogen atom.
The French Revolution brought changes to Coulomb’s professional life. His role in the Académie des Sciences ended when it was closed. His contributions to the weights and measures committee and the supervision of the water supply ceased during the revolution, but in later years he was able to resume some of this work. In June of 1806 he contracted a fever that caused his death in August 6 , but Coulomb’s name lives on in physics. Today, the coulomb is the unit of electric charge, and the scattering observed by Rutherford is Coulomb scattering.
Richard Williams
The author is a regular contributor to This Month in Physics History.
C. Stewart Gillmor, Coulomb and the Evolution of Physics and Engineering in Eighteenth Century France (Princeton University Press, Princeton, New Jersey, 1971).
C. A. Coulomb, Premiere Memoire sur l’electricite et le Magnetisme, Histoire de l’Academie Royale des Sciences , 569-577 (1785). An English translation of this article is available by searching on “Bucciarrelli translation of Coulomb's first memoir” with either Google Search or Bing.
C. A. Coulomb, Seconde Memoire sur l’electricite et le Magnetisme , Histoire de l’Academie Royale des Sciences , 578-611 (1785).
C. A. Coulomb, Quatrieme Memoire sur l’electricite et le Magnetisme, Histoire de l’Academie Royale des Sciences , 67-77 (1785).
Join your Society
If you embrace scientific discovery, truth and integrity, partnership, inclusion, and lifelong curiosity, this is your professional home.
Charles Augustin de Coulomb and the Electrostatic Force
Charles-Augustin de Coulomb (1736 – 1806)
On June 14 , 1736 , French physicist Charles Augustin de Coulomb was born. He is best known for developing Coulomb’s law , the definition of the electrostatic force of attraction and repulsion, but also did important work on friction. The SI unit of electric charge , the coulomb , was named after him.
Charles Augustin de Coulomb – Early Years
Charles Augustin de Coulomb received a good education in mathematics , astronomy , chemistry and botany since both sides of his family were respected and quite wealthy which allowed Coulomb to be raised as a child of privilege. He went to school in the Collège Mazarin in Paris, but, after his father had made some poor decisions, the family faced financial difficulties and Coulomb moved along with his father to Montpelier , where he joined the Academy of Sciences and managed to present several papers, focusing mostly on topics in astronomy and mathematics . He began studying there in early 1760 and graduated at the end of 1761, beginning his career in government service as a lieutenant in the Corps de Génie, where Coulomb performed tasks as a civil engineer, for example in the repair of fortifications. He was initially stationed in Brest and from 1764 in Martinique in the Caribbean, which had been temporarily conquered by the English in 1762 and was to be more strongly fortified after being returned by France. Until 1772, Coulomb directed the construction of the new Fort Bourbon. The fruit of this work was also a treatise on structural analysis, which he published in the treatises of the French Academy of Sciences (Académie des sciences) in 1773 after his return to France, and which brought him to the attention of scientists such as his former teacher Charles Bossut.
Engineering and Mechanics
Coloumb began to focus more and more in engineering and mechanics, which provided him with a firm foundation on which his later theoretical efforts were built. He submitted his first treatise to the Academy of Sciences in Paris in 1773 , and many more would follow on topics ranging from mathematical solutions of engineering problems to studies of friction , elasticity , electricity and magnetism . While stationed in Cherbourg, Coulomb wrote a treatise on the compass that won him a prize from the Paris Academy in 1777. The treatise was also significant because it dealt with the torsion balance (and, in general, the torsion of thin threads), which would later become the basis of his experiments to measure even weak forces. After a visit to Rochefort in the late 1770s , Coloumb was promoted to a Captain and employed at La Rochelle , the Isle of Aix and Cherbourg . In this period, Coulomb discussed his experiments with electrostatic forces and explained the inverse square law that they led Coulomb to posit.
Coulomb electric balance, torsion balance
The Inverse Square Law
Similar to Isaac Newton’s inverse square law of gravitational force , Coulomb’s law states that the electric force between charged objects inversely depends upon the distance between the objects. That is, like gravity, the electric force acts in a line between two objects and decreases with the square of the distance between them. The primary difference between the law for gravity and that for electric force is that gravitation is influenced by the mass of the objects, whereas Coulomb’s law depends upon the charge of the objects involved. When the objects in question are both positively or both negatively charged, the forces between them are repulsive, but attractive forces arise between objects carrying opposing charges [1].
A Wooden Fortress
In 1779, he was sent to Rochefort to help the Marquis de Montalembert, who was pursuing new ideas in fortress construction that deviated from the classical line of Vauban,[ 9 ] to build a fort entirely of wood. At the Rochefort shipyards, he conducted experiments that led to his treatise Theorie des machines simples, in which he developed his theory of frictional forces. It earned him the Grand Prize of the Paris Academy of Sciences in 1781 and led to his admission to the Academy, which made Coulomb independent.
Later Years
After the outbreak of the French Revolution in 1789, Coulomb lost or resigned many of his posts. In 1791 he left the Corps de Genie. After the Academy of Sciences was dissolved in 1793, he retired to the countryside in Blois , where he owned a house and continued his experiments. It was not until 1795 that he returned to Paris with the founding of the Institut de France, which replaced the Academy, and his election as a member of the Institute. Napoleon brought him back into the civil service from 1802 to 1806 as supervisor of the country’s education system, promoting the establishment of new grammar schools (Lycée) throughout France. Charles-Augustin de Coulomb passed away on August 23 , 1806 .
References and Further Reading:
- [1] Charles Augustin de Coulomb at the University of Florida
- [2] Charles Augustin de Coulomb at the University of St Andrews
- [3] André Marie Ampére and Electromagnetism , SciHi Blog
- [4] John J. O’Connor, Edmund F. Robertson: Charles Augustin de Coulomb . In: MacTutor History of Mathematics archive
- [5] Chisholm, Hugh, ed. (1911). “ Coulomb, Charles Augustin “. Encyclopædia Britannica . 7 (11th ed.). Cambridge University Press.
- [6] Works by or about Charles-Augustin de Coulomb at Internet Archive
- [7] Charles Augustin de Coulomb, Théorie des machines simples (1821)
- [8] Charles Augustin de Coulomb, Collection de mémoires relatifs à la physique (1884)
- [9] Vauban or the Art of Fortress Construction , SciHi Blog
- [10] Charles Augustin de Coulomb at Wikidata
- [11] Walter Lewin, 8.02x – Lect 1 – Electric Charges and Forces – Coulomb’s Law – Polarization , Lectures by Walter Lewin. They will make you ♥ Physics. @ youtube
- [12] Timeline of French physicists , via Wikidata and DBpedia
Tabea Tietz
Related posts, pierre simon de laplace and his true love for astronomy and mathematics, cardinal richelieu and the académie francaise, pierre bouguer – child prodigy and ‘father of photometry’, around the world in 80 days, one comment.
Pingback: Whewell’s Gazette: Year 3, Vol. #44 | Whewell's Ghost
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Further Projects
- February (28)
- January (30)
- December (30)
- November (29)
- October (31)
- September (30)
- August (30)
- January (31)
- December (31)
- November (30)
- August (31)
- February (29)
- February (19)
- January (18)
- October (29)
- September (29)
- February (5)
- January (5)
- December (14)
- November (9)
- October (13)
- September (6)
- August (13)
- December (3)
- November (5)
- October (1)
- September (3)
- November (2)
- September (2)
Legal Notice
- Privacy Statement
Charles de Coulomb
Claimed by Alanna Carnevale
Charles-Augustin de Coulomb was a French physicist most well known for the discovery of Coulomb's Law and his work with friction. The SI unit for electric charge known as the coulomb was named after him.

- 1.1 University Education and Career
- 2.1 Coulomb’s Law
- 3 Interesting Facts
- 5.1 External links
Personal Life
Coulomb was born in Angoulême, France, on June 14, 1736 to Henry Coulomb and Catherine Bajet. Both his parents were rather wealthy, as his father was a lawyer, and his mother came from a well-established family. After being raised in Angoulême, Coulomb and his family moved to Paris where he would enter college.
When Henry Coulomb made poor financial choices, lost all of his money, and moved to Montpellier, Charles de Coulomb had to make the decision of whether to move with him or stay in Paris with his mother. Coulomb decided to live with his father after a disagreement with mother about his future career. In March 1757, he joined the Society of Sciences in Montpellier to whom which he would read many papers on mathematics as well as astronomy. Around February 1760, when Coulomb was entering the École du Génie at Mézières he made many significant friends including Bossut and Borda.
During the beginning of the French Revolution, Coulomb was in intense scientific study. He was expelled from the government as many French aristocrats were at the time, and he retired from the Corps du Genie. Coulomb married the mother of his two sons in 1802, Louise Francoise LeProust Desormeaux. Due to work in the West Indies, Coulomb suffered from sicknesses that caused him to be in poor health for the rest of his life. He fell ill with a fever in 1796 and passed away on August 23, 1806 in Paris at the age of 70.
University Education and Career
Charles de Coulomb started his college education at Collège Mazarin in Paris where he learned language, literature, philosophy, mathematics, astronomy, chemistry, and botany. He was most interested in mathematics and astronomy. After moving to Montpellier Coulomb realized he wanted to enter the École du Génie at Mézières but first needed to be tutored so that he could pass the entrance exams. Coulomb studied Camus’s Cours de mathématiques and passed the exams. He graduaded from École du Génie at Mézières in November 1761.
As both a trained engineer and a Corps du Génie lieutenant, Coulomb would be take on a variety of career positions. He went from Brest in February of 1764 to Martinique in the West Indies where he would contribute to making the island safer by building a fort named Fort Bourbon. Coulomb also spent time in Bouchain as well as Cherbourg where Coulomb submitted a memoir to the appointed comptroller general Robert-Jacques Turgot that regarded possible reformations. Coulomb’s political views were present in this memoir and he included how he wanted the state and the individual to be equal. Coulomb also was stationed in Rochefort in 1779 where he would construct a wood fort near Ile d'Aix.
Besides all of this work, Coulomb also participated in fields such as education and hospital reformations. He became in charge of watching over royal fountains and a large part of the Paris water supply in 1784. Coulomb spent his last years working with education as inspector general of pubic instruction.
Scientific Contribution
Coulomb wrote many important works throughout his life. One of these entailed a combination of mathematics and physics, which would determine the influence of friction and cohesion in statics problems. It is notable that Coulomb used variations of calculus to solve engineering problems.
Coulomb wrote a famous memoir on the magnetic compass, which contained his first work on the torsion balance. His use of torsion balance would help many physicists with their works in years to come.
Coulomb also used shipyards in Rochefort as laboratories to come up with his major work on friction titled ‘’Théorie des machines simples’’. This work, consisting of both static and dynamic friction of sliding surfaces and friction in bending of cords and in rolling, caused him to win the Grand Prix in 1781 from the Académie des Sciences.
Coulomb gave twenty-five memoirs to the Académie des Sciences between 1781 and 1806 and contributed to work with 310 committees.
Coulomb’s Law
Coloumb used a torsion balance to examine repulsion and attraction forces of charged particles. This torsion balance he used includes an insulating rod with a metal-coated ball attached to an end suspended from the middle of it by a silk thread. After charging the ball with static electricity, a second charged ball would be brought near it. The two balls repelled one another. Through much analysis, he discovered that the magnitude of the electric force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between the charges. The force is along the straight path between them, and if the charge of the two particles have the same sign then the force is repulsive while if they have different signs the force is attractive.
[math]\displaystyle{ \ |F|=\frac{1}{4 \pi \epsilon_0 } \frac{|q_1q_2|}{r^2},\text{where } \frac{1}{4 \pi \epsilon_0 } \text{is approximately } 9*10^{9}, \text{q1 and q2 are the charges of the particles, and r is the magnitude of the distance between the two.} }[/math]
The first part of this equation, [math]\displaystyle{ \ \frac{1}{4 \pi \epsilon_0 } }[/math] , often denoted as [math]\displaystyle{ \ k_e }[/math] is Coulomb's constant which is a proportionality factor.
Interesting Facts
Coulomb leaves a legacy as a pioneer in geotechnical engineering for his contribution to retaining wall design.
Charles de Coulomb's name is one of the 72 names inscribed on the Eiffel Tower.
Coulomb explained the laws of attraction and repulsion between magnetic poles and electric charges, but he did not find any relationship between the two. He believed that the attraction and repulsion were brought on by different kinds of fluids.
Coulomb worked strenuously on friction of machinery, windmills, and the elasticity of metal and silk.
Charles de Coulomb's Wikipedia Page
YouTube Video about Coulomb's Law and Electric Fields
External links
http://www.biography.com/people/charles-de-coulomb-9259075
http://www.10-facts-about.com/Charles-Augustin-de-Coulomb/id/1524
http://www-history.mcs.st-and.ac.uk/Biographies/Coulomb.html
http://www.britannica.com/biography/Charles-Augustin-de-Coulomb
Navigation menu
Charles-Augustin de Coulomb

Charles-Augustin de Coulomb (June 14, 1736 – August 23, 1806), a French engineer and physicist, discovered the relationship between the force that exists between two electrically charged bodies and the distance that separates them, known as Coulomb's Law . He also studied frictional forces, and used an advanced mathematical technique called the variational calculus to analyze the forces on materials used in construction.
- 1.1 Early Life
- 1.2 Professional career and early research
- 1.3 Inverse square law for electrically charged bodies
- 2 Scientific accomplishments
- 3 Coulomb's law
- 5 References
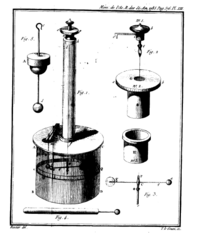
Coulomb was born in Angoulême, France . He was the son of Henry Coulomb and Catherine Bajet, both of whose families were situated in the higher strata of French society.
While still quite young, Coulomb's family moved to Paris , where he received instruction in the arts and sciences at the College Mazarin. Coulomb and his father moved to Montpellier after his father suffered a financial setback. During this time, Coulomb submitted some of his first work to the Society of Sciences in that city. He returned to Paris for tutoring, and passed the exams necessary for his entry to the Ecole du Genie in the town of Mezieres in 1760.
Professional career and early research
After graduating in 1761, he assumed the position of lieutenant in the French army as a military engineer. His first major project was to strengthen fortifications on the island of Martinique in the French West Indies . His health suffered setbacks during the three years he spent at this task that would affect him for the rest of his life.
In a 1773 paper, Coulomb applied his mathematical skills to a number of construction problems using the variational calculus. Some of the techniques he wrote about, including a sliding-wedge theory of soil mechanics, are still in use today. Five years later, he submitted a paper to the Academie des Sciences, reporting research he had done on the magnetic compass using a tortion balance, a device that uses the force generated from twisting a thin thread to measure other forces. For this work he shared the Grand Prix of the Academie des Sciences in 1777.
Inverse square law for electrically charged bodies
In 1779, Coulomb began work on the construction of a fort in Rochefort. But he also found time to experiment on mechanics, writing "The Theory of Simple Machines," for which he was awarded the Grand Prix. He then turned his attention to researches on electricity and magnetism, submitting an average of a paper a year over seven years beginning in 1785. It was during this time that he showed, using his previously perfected tortion balance, that the electrical force between charged bodies varies inversely as the square of the distance between them, and is porportional to the charge of each, being an attractive force for opposite charges, and a repelling force for charges of the same kind. He also demonstrated that non-conductors pass electricity to some extent.
From the early 1780s to the first decade of the 1800s, Coulomb continued to be involved with public life as the turbulent politics of those times permitted. He reported on canal and harbor works in Brittany in 1784, was placed in charge of the King's fountains the same year. He also played a role in ensuring Paris's water suppply. In the midst of the French revolution, he retired from the Corps du Genie, and continued his research from a home in Blois. In 1790, his first son was born to Louise Francoise LeProust Desormeaux, who he would marry in 1802 after the birth of the couple's second son. From that time until 1806, he was, as inspector general of public instruction, instrumental in establishing lycees throughout the country. Coulomb, being of ill health in his later years, died in 1806.
Scientific accomplishments
Coulomb is distinguished in the history of mechanics and of electricity and magnetism . In 1779, he published an important investigation of the laws of friction ( Théorie des machines simples, en ayant égard au frottement de leurs parties et à la roideur des cordages ), which was followed 20 years later by a memoir on viscosity .
In 1784, his Recherches théoriques et expérimentales sur la force de torsion et sur l'élasticité des fils de metal (Histoire de l’Académie Royale des Sciences, 229-269, 1784) appeared. This memoir contained a description of different forms of his torsion balance. He used the instrument with great success for the experimental investigation of the distribution of charge on surfaces, and of the laws of electrical and magnetic force.
In 1785, Coulomb presented his three reports on Electricity and Magnetism :
- Premier Mémoire sur l’Electricité et le Magnétisme , Histoire de l’Académie Royale des Sciences, 569-577, 1785. In this publication Coulomb describes “How to construct and use an electric balance (torsion balance) based on the property of the metal wires of having a reaction torsion force proportional to the torsion angle.” Coulomb also determined experimentally the law of the forces that “two bodies electrified of the same kind of electricity exert on each other.”
- Sécond Mémoire sur l’Electricité et le Magnétisme , Histoire de l’Académie Royale des Sciences, 578-611, 1785. In this publication Coulomb carries out the “determination according to which laws both the Magnetic and the Electric fluids act, either by repulsion or by attraction.”
- Troisième Mémoire sur l’Electricité et le Magnétisme , Histoire de l’Académie Royale des Sciences, 612-638, 1785. “On the quantity of Electricity that an isolated body losses in a certain time period , either by contact with less humid air, or in the supports more or less idio-electric.”
Coulomb explained the laws of attraction and repulsion between electric charges and magnetic poles, although he did not find any relationship between the two phenomena. He thought that the attraction and repulsion were due to different kinds of fluids.
The SI unit of charge, the coulomb, and Coulomb's law are named after him.
Coulomb's law
Using a torsion balance, Coulomb was able to measure the electrostatic force between two electrically charged objects of small dimensions. His observations led him to discover a mathematical relationship that came to be called Coulomb's law . This law may be stated as follows: the magnitude of the electrostatic force between two point charges is directly proportional to the magnitudes of each charge and inversely proportional to the square of the distance between the charges. The formula to Coulomb's Law is of the same form as Newton's Gravitational Law: The electrical force of one body exerted on the second body is equal to the force exerted by the second body on the first. To calculate the magnitude of the force, it may be easiest to consider the simplified, scalar version of the law:

In cgs units, the unit charge, esu of charge or statcoulomb, is defined so that this Coulomb force constant is 1.

- Electricity
References ISBN links support NWE through referral fees
- Abbott, David, (ed.). 1984. The Biographical Dictionary of Scientists . New York: Peter Bedrick. ISBN 0195210832 .
- Asimov, Isaac. 1982. Asimov's Biographical Encyclopedia of Science and Technology . 2nd ed. New York: Doubleday. ISBN 0385177712 .
- Ferguson, Pamela. 2002. World Book's Biographical Encyclopedia of Scientists . 8th ed. Chicago: World Book. ISBN 0716676001 .
- Gillispie, Charles Coulston. 1975. Dictionary of Scientific Biography . New York: Scribner. ISBN 0684101211 .
This article incorporates text from the Encyclopædia Britannica Eleventh Edition , a publication now in the public domain .
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards . This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Charles-Augustin_de_Coulomb history
- Coulomb's_law history
The history of this article since it was imported to New World Encyclopedia :
- History of "Charles-Augustin de Coulomb"
Note: Some restrictions may apply to use of individual images which are separately licensed.
- Physical sciences
- Biographies of Scientists and Mathematicians
- Pages using ISBN magic links
CERN Accelerating science
First observations of the spontaneous discharge of an electrometer.
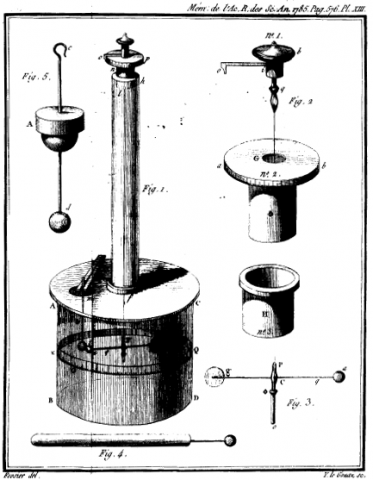
In 1785, the French physicist Charles Augustin de Coulomb made three reports on electricity and magnetism to France’s Royal Academy of Sciences. His third paper described an experiment with a torsion balance, which showed that the device would spontaneously discharge due to the action of the air rather than defective insulation.
In 1850, Italian physicist Cano Matteucci and later British physicist William Crookes in 1879 showed that the rate of spontaneous discharge decreased at lower atmospheric pressures. The search for an explanation for the nature of this spontaneous discharge paved the way for the discovery of cosmic rays – high-energy particles from outer space.
Read more: Extract from Mémoires sur l'électricité et le magnétisme (1785-89) by Charles Augustin de Coulomb.
de Coulomb, Charles-Augustin
- Living reference work entry
- First Online: 18 October 2019
- Cite this living reference work entry

- Holm Altenbach 3
24 Accesses
Charles-Augustin de Coulomb (June 14, 1736, in Angoulême, France; August 23, 1806, in Paris, France) was a military engineer and physicist.

Coulomb’s family moved to Paris early in his childhood, and he got his education at the Collège Mazarin. His main focus was on philosophy, language, and literature, but he also received a good education in mathematics, astronomy, chemistry, and botany. With respect to his father’s financial setback, he had to leave Paris and moved to Montpellier. Coulomb wrote his first publication in that time and submitted to the Society of Sciences in Montpellier. After his return to Paris, he passed the exams for the École royale du génie de Mézières in 1760. He graduated in 1761 and joined the French army as an engineer in the rank of lieutenant.
Professional Carrier
After graduation he served over the next 20 years in the French army. He was sent to various locations and worked as an engineer with the main focus on...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Author information
Authors and affiliations.
Fakultät für Maschinenbau, Institut für Mechanik, Otto-von-Guericke-Universität Magdeburg, Magdeburg, Germany
Holm Altenbach
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Holm Altenbach .
Editor information
Editors and affiliations.
Otto-von-Guericke University Magdeburg, Chair of Engineering Mechanics, Institut, Magdeburg, Sachsen-Anhalt, Germany
Fakultät für Maschinenbau, Hochschule Esslingen, Esslingen am Neckar, Baden-Württemberg, Germany
Andreas Öchsner

Section Editor information
Rights and permissions.
Reprints and permissions
Copyright information
© 2019 Springer-Verlag GmbH Germany, part of Springer Nature
About this entry
Cite this entry.
Altenbach, H. (2019). de Coulomb, Charles-Augustin. In: Altenbach, H., Öchsner, A. (eds) Encyclopedia of Continuum Mechanics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53605-6_328-1
Download citation
DOI : https://doi.org/10.1007/978-3-662-53605-6_328-1
Published : 18 October 2019
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-662-53605-6
Online ISBN : 978-3-662-53605-6
eBook Packages : Springer Reference Engineering Reference Module Computer Science and Engineering
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Vera Rubin and Dark Matter
The emergence of the age of ai, openmind books, scientific anniversaries, fungi: the next great pandemic scourge, featured author, latest book, the olympus of electricity: the geniuses who gave us energy.
Nowadays it would be difficult for us to live without artificial light, without taps that supply us with hot and cold water, without heating or without vehicles that transport us from one place to another. But even if we associate these comforts with different energy sources, all of them depend irremediably on one and the same technological advance: electricity. Without the mastery, production and distribution of this energy, we would return to a medieval world based on animal traction and fire.
But electricity is not only the foundational pillar of technological civilisation, but also one of the best examples of scientific progress as a collective work, fruit of the contributions of numerous fathers . Here we review the contributions of the principal geniuses who deserve a special place in the Olympus of the history of electricity.
William Gilbert
Static electricity has been known since ancient times, but it was in 1600 that the British man of science William Gilbert (24 May 1544 – 30 November 1603) first approached its scientific study. Fulfilling the commission of Queen Elizabeth I of England to study the functioning of the compass, Gilbert discovered that magnetism was related to the attraction that amber exerted on small objects when rubbed. Gilbert coined the term for this phenomenon electricus , from the Greek word for amber, elektron .

Benjamin Franklin
After Gilbert’s studies, other men of science began to investigate the properties of this strange electrical matter that could be created, stored in so-called Leyden bottles, and transmitted through wires. However, evidence was lacking that this mysterious fluid existed outside of laboratories. It was American polymath Benjamin Franklin (October 18, 1785 – November 5, 1788) who, in 1752, with his famous kite experiment , demonstrated that the energy of storms and the energy of Leyden’s bottles were the same thing, thereby establishing the science of electricity.

Luigi Galvani
In the 1780s, the Italian scientist Luigi Galvani (9 September 1737 – 4 December 1798) and his wife, Lucia Galeazzi , described “animal electricity” as the power that nerves transmitted through a liquid medium to trigger muscle movement. The idea of galvanism attracted popular interest in the electricity of living beings, to the point of inspiring writer Mary Wollstonecraft Shelley to write her work Frankenstein or the modern Prometheus . The work of the Galvanis laid the foundations for the later knowledge of the electrophysiology of the nervous system.

Alessandro Volta
A contemporary and friend of Galvani’s, although a rival in the scientific field, the also Italian Alessandro Volta disputed the theory of animal electricity , claiming that the current observed in his colleague’s experiments with frog legs had an external origin. When Volta replaced biological material with cardboard soaked in a salt solution, he invented the first battery, a constant source of electrical current that did not depend on electrostatic generation.
The invention of the voltaic battery in 1799 offered scientists a valuable tool for the study of electricity. In recognition of his work, his name was given to the unit of electrical potential, the volt.

Charles-Augustin de Coulomb
In the second half of the 18th century, scientists such as Joseph Priestley or Henry Cavendish, both English, began to observe experimentally that the force of attraction or repulsion between two charges depended on the magnitude of the charges and was inversely proportional to the distance between them, like the gravitational force described by Isaac Newton. It was French engineer and physicist Charles-Augustin de Coulomb (14 June 1736 – 23 August 1806) who in 1785 formulated the law that bears his name . In 1908 the unit of charge, the coulomb, was named in his honour.

André-Marie Ampère
If Coulomb’s impetus was decisive for the formulation of electrostatics, it was his compatriot André-Marie Ampère who laid the foundations of electrodynamics. Based on the earlier work of Danish physicist and chemist Hans Christian Ørsted, in the 1820s Ampère began to give physical and mathematical form to the force of attraction or repulsion between two parallel wires conducting electric current. Ampère’s force law opened the way to the understanding and mathematical definition of electromagnetism, which in 1881 was recognised by assigning the name of ampere to the unit of measure of electric current.
While Ampère was investigating the force in action between two electric wires, German physicist and mathematician Georg Ohm (16 March 1789 – 6 July 1854) was using Volta’s batteries and self-designed devices to study how the current (I) varied according to the applied voltage (V) and the resistance of the circuit (R). This issue had already interested scientists like Cavendish, who used his body to close the circuit and experience the force of the electric shock in each case. The law summarising Ohm’s results, I = V/R, may seem almost obvious today, and yet the publication of its original formulation in 1827 aroused more suspicion than applause. Subsequently, Ohm’s contribution would be embodied in the name of the unit of electrical resistance, the ohm.

Michael Faraday
During the nineteenth century, the era of the flourishing of electrical science, there were numerous scientists dedicated to unravelling the physical and mathematical principles of electricity. But someone had yet to turn all that knowledge into practical technology, and in this field Englishman Michael Faraday (22 September 1791 – 25 August 1867) stood out with his development of what would become the electric motor. However, Faraday did not limit himself to invention, and his observations would provide the material to build a complete theory of electromagnetism . He was a self-taught scientist whose humble origins led him to work for chemist Humphry Davy, not only as an assistant but also as a valet. Faraday’s genius led to his fame surpassing that of his mentor, and today the unit of electrical capacitance bears his name, the farad.

James Clerk Maxwell
It can be said that the finishing touch to the golden age of electrical science was the work of Scottish scientist James Clerk Maxwell (13 June 1831 – 5 November 1879), who between 1861 and 1862 published a set of equations that turned Faraday’s intuitive observations into a complete theory of electromagnetism. Maxwell’s equations, eventually reduced to four, collected and summarised all the work of his predecessors to serve as tablets of law under the unified realm of the electromagnetic field. In turn, and given that science is usually a collective construction without beginning or end, Maxwell’s equations would be in the following century one of the starting points for the birth of another new science: quantum physics.

Javier Yanes
Related publications.
- Olbers' Paradox and Electromagnetic Waves
- The Electron Revolution
- Millikan, the First Physicist to See the Electron
More about Science
Environment, leading figures, mathematics, scientific insights, more publications about ventana al conocimiento (knowledge window), comments on this publication.
Morbi facilisis elit non mi lacinia lacinia. Nunc eleifend aliquet ipsum, nec blandit augue tincidunt nec. Donec scelerisque feugiat lectus nec congue. Quisque tristique tortor vitae turpis euismod, vitae aliquam dolor pretium. Donec luctus posuere ex sit amet scelerisque. Etiam sed neque magna. Mauris non scelerisque lectus. Ut rutrum ex porta, tristique mi vitae, volutpat urna.
Sed in semper tellus, eu efficitur ante. Quisque felis orci, fermentum quis arcu nec, elementum malesuada magna. Nulla vitae finibus ipsum. Aenean vel sapien a magna faucibus tristique ac et ligula. Sed auctor orci metus, vitae egestas libero lacinia quis. Nulla lacus sapien, efficitur mollis nisi tempor, gravida tincidunt sapien. In massa dui, varius vitae iaculis a, dignissim non felis. Ut sagittis pulvinar nisi, at tincidunt metus venenatis a. Ut aliquam scelerisque interdum. Mauris iaculis purus in nulla consequat, sed fermentum sapien condimentum. Aliquam rutrum erat lectus, nec placerat nisl mollis id. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Nam nisl nisi, efficitur et sem in, molestie vulputate libero. Quisque quis mattis lorem. Nunc quis convallis diam, id tincidunt risus. Donec nisl odio, convallis vel porttitor sit amet, lobortis a ante. Cras dapibus porta nulla, at laoreet quam euismod vitae. Fusce sollicitudin massa magna, eu dignissim magna cursus id. Quisque vel nisl tempus, lobortis nisl a, ornare lacus. Donec ac interdum massa. Curabitur id diam luctus, mollis augue vel, interdum risus. Nam vitae tortor erat. Proin quis tincidunt lorem.
When Music becomes a Quasi-Philosophical Exercise
Do you want to stay up to date with our new publications.
Receive the OpenMind newsletter with all the latest contents published on our website
OpenMind Books
- The Search for Alternatives to Fossil Fuels
- View all books
About OpenMind
Connect with us.
- Keep up to date with our newsletter

14 Intriguing Facts About Charles-Augustin De Coulomb
Written by Carri Santana
Modified & Updated: 30 May 2024
Reviewed by Sherman Smith

Charles-Augustin de Coulomb was a renowned French physicist and engineer who made significant contributions to the field of electromagnetism. Born on June 14, 1736, in Angoulême, France, Coulomb dedicated his life to understanding the forces that govern the interaction between charged particles.
Throughout his career, Coulomb conducted groundbreaking experiments and formulated fundamental laws that continue to shape our understanding of electricity and magnetism to this day. His discoveries range from the development of Coulomb’s Law, which describes the electrostatic force between charged objects, to his work on torsion balance, which allowed him to measure the strength of electric forces accurately.
This article delves into the intriguing life and scientific accomplishments of Charles-Augustin de Coulomb. From his early education to his groundbreaking experiments and the impact of his work on modern physics, we will explore 14 fascinating facts that showcase the brilliance and lasting legacy of this remarkable scientist.
Key Takeaways:
- Coulomb’s work in electromagnetism laid the foundation for modern electrical theory, including Coulomb’s Law and the concept of electrical capacitance. His discoveries continue to shape our understanding of electricity.
- Despite not receiving recognition during his lifetime, Coulomb’s research influenced the development of electrical engineering and inspired future scientists. His legacy lives on as a source of inspiration for aspiring physicists.
Coulomb is known for his significant contributions to the field of physics.
Charles-Augustin de Coulomb was a French physicist and engineer who made groundbreaking discoveries in the realm of electromagnetism. His work laid the foundation for modern-day electrical theory.
Coulomb’s Law revolutionized the understanding of electrostatics.
Coulomb’s Law states that the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This law is fundamental in studying the behavior of electrically charged objects.
Coulomb conducted extensive experiments on friction and static electricity.
Coulomb’s experiments involved measuring the forces of attraction and repulsion between charged objects, furthering our understanding of the nature of electricity and the effect of friction on electrical charges.
Coulomb invented the torsion balance.
The torsion balance, also known as the Coulomb balance, was a device created by Coulomb to measure the forces of repulsion and attraction between charged objects accurately. This invention was pivotal in conducting precise experiments on electrostatics.
Coulomb was the first to quantify the concept of electrical capacitance.
Coulomb developed the concept of capacitance , which measures an object’s ability to store an electric charge. His work laid the groundwork for the development of capacitors widely used in modern electronic devices.
Coulomb discovered the laws governing the behavior of magnetism.
In addition to his contributions to electrostatics, Coulomb also explored the principles of magnetism. He established mathematical relationships that govern the force and behavior of magnets.
The unit of electric charge, the coulomb, is named after Charles-Augustin de Coulomb.
In recognition of his significant contributions to the field of electromagnetism, the International System of Units (SI) named the unit of electric charge after him. One coulomb is equal to the charge carried by approximately 6.24 x 10^18 electrons.
Coulomb’s discoveries paved the way for the development of electrical engineering.
By unraveling the fundamental properties of electrical charges, Coulomb’s work laid the foundation for the field of electrical engineering. His principles and laws are still actively used in the design and development of electrical systems and devices.
Charles-Augustin de Coulomb had a diverse career.
Before devoting himself to scientific pursuits, Coulomb served as a military engineer in France. His background in engineering provided him with a strong foundation for his later scientific endeavors.
Coulomb’s contributions were not widely recognized during his lifetime.
Although Coulomb’s work was groundbreaking, it was not until after his death that his contributions were fully acknowledged. Today, he is considered one of the great pioneers of modern physics .
Coulomb’s findings influenced other notable scientists.
Coulomb’s laws and principles had a profound impact on other renowned physicists, including Michael Faraday and James Clerk Maxwell, who further expanded upon his work and made significant advancements in the field of electromagnetism.
Coulomb’s research extended beyond physics.
While best known for his contributions to physics, Coulomb also made important advancements in fields such as mechanics and engineering. He published numerous papers on a wide range of scientific topics.
Charles-Augustin de Coulomb’s legacy continues to inspire aspiring scientists.
Today, Coulomb’s work serves as a source of inspiration for young scientists around the world. His pioneering spirit and dedication to scientific inquiry have left an indelible mark on the world of physics.
Coulomb’s principles form the basis for our understanding of electricity and magnetism.
The principles and laws established by Charles-Augustin de Coulomb continue to be essential in the field of electromagnetism. His work provides the framework for understanding the behavior of electrical charges and magnetic fields.
In conclusion, Charles-Augustin de Coulomb was a remarkable figure in the field of physics. His contributions to the study of electromagnetism and friction laid the foundation for many modern scientific principles. From his groundbreaking experiments with electrical charges to his mathematical formulation of Coulomb’s Law, Coulomb’s work continues to shape our understanding of the physical world.Not only was Coulomb a brilliant scientist, but he was also a dedicated engineer and a respected military officer. His diverse background and expertise allowed him to explore various scientific phenomena and make significant discoveries that still impact the field today.Through his experiments and calculations, Coulomb played a pivotal role in advancing our understanding of the forces that govern the behavior of electric charges. His work demonstrates the power of scientific inquiry and the pursuit of knowledge.In summary, Charles-Augustin de Coulomb’s life and work serve as a testament to the extraordinary contributions that individuals can make to the world of science and innovation.
1. Who was Charles-Augustin de Coulomb?
Charles-Augustin de Coulomb was a French physicist, engineer, and military officer. He is best known for his work in the field of electromagnetism and the formulation of Coulomb’s Law.
2. What is Coulomb’s Law?
Coulomb’s Law states that the force between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
3. What were Coulomb’s major contributions to physics?
Coulomb made significant contributions to several areas of physics, including the study of electrical charges, friction, and magnetism. His experiments and calculations laid the foundation for our understanding of electromagnetism.
4. Was Coulomb only a scientist?
No, Coulomb had a diverse background. He was also an engineer and a military officer. His engineering expertise allowed him to apply his scientific knowledge to practical innovations.
5. How did Coulomb’s work impact the field of physics?
Coulomb’s work revolutionized the study of electromagnetism and laid the foundation for future advancements in the field. His principles and laws are still widely used and form an integral part of modern physics.
Coulomb's groundbreaking work in electromagnetism continues to shape our understanding of the universe. His law of electrostatic forces, magnetic repulsion principles, and pioneering research into electrostatics laid the foundation for modern physics. Curious minds eager to explore Coulomb's scientific legacy further should consider delving into the astounding facts surrounding his eponymous law , mind-blowing insights into magnetic repulsion , and captivating details about his electrostatics research . These fascinating topics promise to illuminate the genius behind one of history's most influential physicists, offering a deeper appreciation for the profound impact Coulomb's discoveries have had on our world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
Share this Fact:
- Browse Collection
- Shared Folders
- Get Involved
- For New Teachers
- Contact the Editors
- About Physics Front
The Physics Front is a free service provided by the AAPT in partnership with the NSF / NSDL .
COMMENTS
Coulomb's law. electricity. Charles-Augustin de Coulomb (born June 14, 1736, Angoulême, France—died August 23, 1806, Paris) was a French physicist best known for the formulation of Coulomb's law, which states that the force between two electrical charges is proportional to the product of the charges and inversely proportional to the ...
Charles-Augustin de Coulomb (/ ˈ k uː l ɒ m,-l oʊ m, k uː ˈ l ɒ m,-ˈ l oʊ m /, KOO-lom, -lohm, koo-LOM, -LOHM; [1] French:; 14 June 1736 - 23 August 1806) was a French officer, engineer, and physicist.He is best known as the eponymous discoverer of what is now called Coulomb's law, the description of the electrostatic force of attraction and repulsion. He also did important ...
Charles-Augustin de Coulomb invented a device, dubbed the torsion balance, that allowed him to measure very small charges and experimentally estimate the force of attraction or repulsion between two charged bodies. ... One was a thorough discussion of Coulomb's experiments with electrostatic forces and a description of the inverse square law ...
By the 1780s, devices to generate, store and detect static electricity had been built, but there was no easy way to gauge amounts of static electrical charges. A French engineer with an interest in electricity and magnetism, Charles-Augustin de Coulomb, developed one of the earliest instruments capable of this feat: the torsion balance.
Charles-Augustin de Coulomb was born in Angoulême, France, on June 14, 1736, and went on to become one of the most important scientists in the early discovery of electricity.
Much of the modern physical description of electrical forces comes from careful experiments done by the French scientist Charles Augustin Coulomb (1736-1806). His parents came from wealthy families living near Montpellier1, and they moved to Paris when Coulomb's father began work there. Coulomb earned a degree at the engineering school at ...
Charles-Augustin de Coulomb (1736 - 1806) On June 14, 1736, French physicist Charles Augustin de Coulomb was born. He is best known for developing Coulomb's law, the definition of the electrostatic force of attraction and repulsion, but also did important work on friction. The SI unit of electric charge, the coulomb, was named after him.
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law [1] of physics that calculates the amount of force between two electrically charged particles at rest. This electric force is conventionally called the electrostatic force or Coulomb force. [2] Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb.
The French physicist Charles Augustin de Coulomb (1736-1806) was famous for establishing the relation for computing the force between electrical charges. He also did pioneering work on sliding and fluid friction. Charles Augustin de Coulomb was born into a distinguished family of Angoulême on June 14, 1736.
Charles-Augustin de Coulomb was a French physicist most well known for the discovery of Coulomb's Law and his work with friction. The SI unit for electric charge known as the coulomb was named after him. Charles-Augustin de Coulomb. Contents. 1 Personal Life. 1.1 University Education and Career;
Charles-Augustin de Coulomb. Charles-Augustin de Coulomb (June 14, 1736 - August 23, 1806), a French engineer and physicist, discovered the relationship between the force that exists between two electrically charged bodies and the distance that separates them, known as Coulomb's Law. He also studied frictional forces, and used an advanced ...
In 1785, the French physicist Charles Augustin de Coulomb made three reports on electricity and magnetism to France's Royal Academy of Sciences. His third paper described an experiment with a torsion balance, which showed that the device would spontaneously discharge due to the action of the air rather than defective insulation.
Experiment 1: Coulomb's Law. Introduction to Coulomb's LawIn 1785 Augustin de Coulomb investigated the attractive and repulsive forces between charged objects, experimentally formulating what is now referred to as Coulomb's Law: \The magnitude of the electric force that a particle exerts on another is directly proportional to the product of ...
Charles-Augustin de Coulomb (June 14, 1736, in Angoulême, France; August 23, 1806, in Paris, France) was a military engineer and physicist. Charles-Augustin de Coulomb. Education. Coulomb's family moved to Paris early in his childhood, and he got his education at the Collège Mazarin. His main focus was on philosophy, language, and ...
This book contains complete and commented translations of the main works of Charles-Augustin de Coulomb (1736-1806) on torsion, electricity, and magnetism. They include the 1777 prize winning work ...
Abstract: Contemporary scholars are engaged in a debate over whether Charles Augustin Coulomb's results that he presented in his 1785 and 1787 memoirs to the Paris Academy of Sciences were attained experimentally or theoretically. In this paper, we study Coulomb's famous 1785 electric torsion balance experiment through analysis of
In June of 1785, Charles Augustin Coulomb, a retired military engineer, announced to the Paris Academy of Sciences that he had devised an innovative experimental appara-tus, the torsion balance, an extremely sensitive instrument able to measure even minute forces to an unprecedented degree of accuracy. With it Coulomb claimed to have demon-
In 1908 the unit of charge, the coulomb, was named in his honour. Portrait of Charles Augustin de Coulomb (1736-1806). Artist: Louis Hierle André-Marie Ampère. If Coulomb's impetus was decisive for the formulation of electrostatics, it was his compatriot André-Marie Ampère who laid the foundations of electrodynamics. Based on the earlier ...
Charles-Augustin de Coulomb was a renowned French physicist and engineer who made significant contributions to the field of electromagnetism. Born on June 14, 1736, in Angoulême, France, Coulomb dedicated his life to understanding the forces that govern the interaction between charged particles. Throughout his career, Coulomb conducted ...
This is a short biography of Charles Augustin de Coulomb, the 18th-century scientist whose experiments with a torsion balance gave rise to Coulomb's Law -- a fundamental principle of physics that defines the electrical force between two charged particles as a predictable mathematical relationship. For a simulation of Coulomb's torsion balance ...
The electrostatic unit (esu) of charge was defined using Coulomb's law, F = q1q2 r2 F = q 1 q 2 r 2, where F F is the force of attraction/repulsion in dynes (the force required at accelerate 1 1 gramme at 1cms−2 1 c m s − 2 ), r r the separation in centimetres and the charge was then in esu or statCoulomb or franklin.
Charles Augustin Coulomb [3] developed experiments of friction in the latter half of the XVIII century at the request of the French . Académie Royale des Sciences. calling for practical rules and data on friction to design machines. Coulomb was also motivated by misapplications for use in marine machinery, controversy and disputed findings [4 ...
Charles-Augustin de Coulomb, född 14 juni 1736 i Angoulême, departementet Charente, död 23 augusti 1806 i Paris, var en fransk ingenjör och fysiker som gjorde viktiga bidrag till förståelsen av mekanik, elektricitet och magnetism.Laddningsenheten coulomb är uppkallad efter honom.. Coulomb ingick tidigt i franska ingenjörskåren och uppehöll sig flera år på Martinique, där han ...