Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment
Roles Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing
Affiliation National Engineering Laboratory for Improving Quality of Arable Land, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing, China
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing
* E-mail: [email protected]
Roles Data curation, Investigation, Writing – review & editing
Roles Conceptualization, Methodology, Writing – review & editing
Roles Conceptualization, Project administration, Supervision, Writing – review & editing
Roles Investigation, Writing – review & editing
Affiliation Qiyang Agro-ecosystem of National Field Experimental Station, Hunan, China
- Ling Li,
- Minggang Xu,
- Mohammad Eyakub Ali,
- Wenju Zhang,
- Yinghua Duan,

- Published: September 13, 2018
- https://doi.org/10.1371/journal.pone.0203812
- Reader Comments
The effects of soil type and organic material quality on the microbial biomass and functional diversity of cropland soils were studied in a transplant experiment in the same climate during a 1-year field experiment. Six organic materials (WS: wheat straw, CS: corn straw, WR: wheat root, CR: corn root, PM: pig manure, CM: cattle manure), and three contrasting soils (Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem) were chosen. At two time points (at the end of the 1st and 12th months), soil microbial biomass carbon (C) and nitrogen (N) (MBC and MBN) and Biolog Ecoplate substrate use patterns were determined, and the average well color development and the microbial functional diversity indices (Shannon, Simpson and McIntosh indices) were calculated. Organic material quality explained 29.5–50.9% of the variance in MBC and MBN when compared with the minor role of soil type (1.4–9.3%) at the end of the 1st and 12th months, and C/N ratio and total N of organic material were the main parameters. Soil properties, e.g., organic C and clay content were the predominant influence on microbial functional diversity in particular at the end of the 12th month (61.8–82.8% of the variance explained). The treatments of WS and CS significantly improved the MBC and microbial functional diversity indices over the control in the three soils in both sampling periods ( P < 0.05). These results suggest that the application of crop straw is a long-term effective measure to increase microbial biomass, and can further induce the changes of soil properties to regulate soil microbial community.
Citation: Li L, Xu M, Eyakub Ali M, Zhang W, Duan Y, Li D (2018) Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 13(9): e0203812. https://doi.org/10.1371/journal.pone.0203812
Editor: Jingdong Mao, Old Dominion University, UNITED STATES
Received: March 9, 2018; Accepted: August 28, 2018; Published: September 13, 2018
Copyright: © 2018 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This work was supported by the National Natural Science Foundation of China (41571298), and the International (Regional) Joint Research Program (41620104006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Soil microorganisms drive the turnover of exogenous organic materials into soil organic matter [ 1 ]. The quality of applied organic materials can regulate microbial abundance and function [ 2 – 4 ]. In China, large amounts of agricultural byproducts are produced because high agricultural productivity is being pursued to meet the food demands of the huge population. In China in 2011, 863 million tons of crop straw and 3 trillion tons of livestock manure were produced [ 5 – 6 ]. These excessive byproducts have created a series of negative environmental effects, such as atmospheric pollution, water eutrophication and so on. In agricultural systems, the return of organic materials to the soil is the most prevalent practice to maintain or improve soil fertility. However, the size and function of microorganisms is different in different soils, for example, high content of soil organic matter is generally associated with high microbial abundance and diversity [ 7 – 8 ]. Increased understanding of the size and function of microorganisms after application of different organic materials in different types of agricultural soil is therefore helpful to clarify the effect of organic materials and soil types on microbial characteristics.
Soil microbial biomass carbon (C) and nitrogen (N) (MBC and MBN) reflect microbial size and soil fertility status, and they act as the living nutrient pool in soil [ 9 ]. Soil microbial functional diversity is linked with the stability of soil microbial communities and levels of soil biodiversity [ 10 ]. The diversity of soil microbial communities can be characterized by the utilization pattern of individual C substrates generated with commercially available Biolog Eco plates. These community-level physiological profiles (CLPPs) have provided a rapid means for evaluating the structure and species composition of soil microbial communities. The average well color development (AWCD) and the functional diversity indices, including Shannon ( H’ ), Simpson ( D ) and McIntosh ( U ) indices, are important diagnostic indicators of soil quality [ 11 ]. Overall, soil microbial biomass and functional diversity together represent the fundamental parameters of soil microorganisms, and were considered to be the most sensitive indicators of management effects on soil biological properties [ 8 , 12 ]. The analysis of soil microbial characteristics can indicate the status of soil fertility and ecosystem function.
The quality of organic materials affects the microbial biomass and community structure [ 13 – 16 ]. Microbial biomass carbon and Shannon’s diversity index after amendment with labile organic materials with low lignin content were significantly higher than that after amendment with recalcitrant organic materials with high lignin content [ 16 – 17 ]. In agricultural systems, the available organic materials generally include crop residues and livestock manures; crop residues are characterized by higher C:N ratio and lower available nutrient content in comparison with manure [ 15 ]. Generally, the microbial biomass or functional diversity after amendment with crop straws was lower than that with manure in agricultural soils because of the low availability of C sources and nutrients in crop residues [ 13 , 15 – 18 ]. To date, most studies of soil microbial characteristics with different organic materials amendment concentrated mainly on a certain soil or different soils under controlled laboratory conditions [ 17 , 19 ]; little information is reported about comparative studies of microbial characteristics dynamics in soils developed from different parent materials after amendment with different organic materials under field conditions.
Soil properties, such as parent material, soil organic matter, pH and clay content can also influence soil microbial biomass and functional diversity [ 19 – 21 ]. Soil parent material provides the basic nutritional environment for development of the microbial community [ 22 – 23 ], and during soil formation the soil microbial communities can be changed [ 19 , 21 ]. Soil organic matter provides energy to microbes, and soil with higher content of SOM generally has higher microbial biomass and functional diversity [ 7 , 17 , 24 – 26 ]. Soil pH plays an important role in shaping microbial community composition [ 27 – 30 ]; soil pH was negatively correlated with soil biomass and positively correlated with AWCD [ 11 , 13 ]. Soil texture can also affect the soil nutrient status and water content, thus affecting the living environment and metabolic activity of microorganisms [ 31 – 32 ]. Ranjard and Richaume (2001) [ 33 ] found that 40–70% of the bacteria were located in the 2–20 and < 2 μm aggregates. Consequently, the comparison of microbial characteristics in different soil types can improve our understanding of the influence of soil properties on microbes.
In China, the Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem are the typical intensive cropland soils. Currently, these Chinese cropland soils have the obvious trend of acidification because of excessive N fertilizer application when compared with those soils 30 years ago [ 34 ]. Ferralic Cambisol is found in the subtropical region with an acidic soil environment [ 35 ], Calcaric Cambisol is found in the warm temperate region with a weak basic or neutral soil environment, and Calcaric Cambiso is found in the cold temperate region with a weak acidic or neutral soil environment. To better compare the effects of exogenous organic materials and soil type on the microbial characteristics and to eliminate the effect of climate factors, Calcaric Cambisol and Luvic Phaeozem were moved to the subtropical region to accentuate the effects of global warming and soil acidification. The objectives of the present study were therefore (1) to explore the changes in microbial biomass and functional diversity during the decomposition of organic materials in different soil types, and (2) to quantify the contributions of soil type and quality of organic materials to microbial biomass and functional diversity.
Materials and methods
Soils collection.
Three typical cropland soils including Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem (FAO classification) were collected from the national long-term monitoring stations of soil fertility which were established by Qiyang Agro-ecosystem of National Field Experimental Station, Henan Academy of Agricultural Sciences and Jilin Academy of Agricultural Sciences, respectively. The Ferralic Cambisol developed from the quaternary red soil was located in Qiyang County, Hunan Province; this region has a subtropical climate, with an annual average temperature of 18°C and an average annual rainfall of 1255 mm. The Calcaric Cambisol developed from alluvial sediments of the Yellow River was located in Yuanyang County, Henan Province; this region has a temperate sub-humid climate, with an annual average temperature of 14.5°C and an average annual rainfall of 450–600 mm. The Luvic Phaeozem derived from the quaternary loess sediments was located in Gongzhuling County, Jilin Province; this region is characterized by temperate sub-humid climate, with an annual average temperature of 4–5°C and an average annual rainfall of 450–600 mm. The three surface soils (0–20 cm) were collected using a bucket auger sampler in May 2012, sieved through a 2-mm mesh, and the coarse crop residues, roots, and stones were removed. The soil physicochemical properties were shown in Table 1 and the average monthly rainfall and temperature of the study site during the sampling period were shown in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0203812.t001
https://doi.org/10.1371/journal.pone.0203812.g001
Preparation of organic material
Six kinds of organic materials were chosen, including wheat ( Triticum aestivum L.) straw (WS), corn ( Zea mays L.) straw (CS), wheat root (WR), corn root (CR), pig manure (PM), and cattle manure (CM). All the organic materials were oven-dried at 60°C, and passed through a 2-mm sieve. The chemical characteristics of these organic materials were shown in Table 2 .
https://doi.org/10.1371/journal.pone.0203812.t002
Experimental design
The experiment was carried out from June 5, 2012 to June 5, 2013 in the long-term experimental station of the Chinese Academy of Agricultural Sciences, Qiyang County (111°52′32″N, 26°45′12″E), Hunan Province. Before the experiment, both Calcaric Cambisol from Zhengzhou and Luvic Phaeozem from Gongzhuling were moved to Qiyang County.
Soil type was the main plot factor and organic material was the subplot factor in a split-plot design with six replicates; three replicates were sampled at the end of the 1st month, and the other three replicates were sampled at the end of the 12th month. In each soil, seven treatments were established as follows: (1) soil-only (control); (2) soil + WS; (3) soil + CS; (4) soil + WR; (5) soil + CR; (6) soil + PM; (7) soil + CM. A total of 126 nylon bags (20 × 15 cm 2 , 0.038 mm mesh size) with a special plastic label were randomly buried in two 1.5 × 1.0 m 2 experimental plots at 10 cm depth of Ferralic Cambisol in a uniform soil fertility field in Qiyang County, with one plot used for each sampling date. In each bag, 200 g (oven-dried basis) experimental soil (Ferralic Cambisol, Calcaric Cambisol, Luvic Phaeozem) was thoroughly mixed with organic material at a ratio of 15 g C kg −1 soil, which was equivalent to 34 t C ha −1 returned to the soil. The amount of different organic materials in each bag was described in S1 Table . During the experimental period, no crops were planted in the plots, and weeds were removed regularly by hand to decrease the effect of weed roots on the nylon bags. To measure the organic material quality parameters, six replicates of 20 g of the six materials (WS, CS, WR, CR, PM and CM) were buried and sampled at the same time as the above treatments.
Sampling and analysis
On the sampling day, each bag was weighed after the attached soil on the outer wall of the bag was carefully removed. Part of the fresh soil in the bag was taken to determine microbial CLPPs, MBC and MBN, while the remaining soil was air-dried to determine soil organic C (SOC), total N, and pH. Total fiber content of organic material (cellulose, hemicellulose and lignin), total organic C, and total organic N were determined.
BIOLOG analysis
Microbial CLPPs in soil were determined by Biolog Eco plates (Biolog, Hayward, CA, USA). Briefly, 5 g of fresh soil was shaken in 45 ml of sterile saline solution (0.85% NaCl w/v) for 30 min at the rate of 180 rpm, and then the mixture was diluted 100-fold. Aliquots of 150 μl of the 10 −3 suspension was incubated in each well of Biolog Eco plates at 28°C and the absorbance was measured at 590 nm with an Emax precision microplate reader (Biolog, Hayward, CA, USA). The readings at 96 h incubation collected by Microlog Rel. 4.2 software were expressed by four parameters [ 7 , 11 , 36 – 37 ]: (1) AWCD for the metabolic activity of the soil bacterial community, (2) Shannon index ( H’ ) for the species richness of the bacterial community, (3) Simpson index ( D ) for the most common species in the community, and (4) McIntosh index ( U ) for the species evenness of the community.
Microbial biomass
Microbial biomass C and N were determined by the fumigation-extraction method [ 39 ]. A 20-g subsample of soil (oven-dried basis) was fumigated by exposing the soil to alcohol-free CHCl 3 vapor in a sealed vacuum desiccator for 24 h. The fumigated soil was evacuated repeatedly in a clean empty desiccator until the odor of CHCl 3 was not detected, and then extracted with 80 ml 0.5 M K 2 SO 4 (soil:K 2 SO 4 = 1:4) for 30 min. The extraction of non-fumigated soil was the same as that of the fumigated soil. Microbial biomass C and N were estimated by the difference between the total organic C or total N in the fumigated and non-fumigated extracts with a conversion factor (K EC ) of 0.38 and (K EN ) of 0.45 [ 40 – 41 ], respectively.
Physicochemical analysis of soil and organic material
Soils and organic materials were analyzed for organic C and total C by dichromate oxidation and total N by Kjeldahl digestion. Soil total P and total K were digested in a nickel crucible with sodium hydroxide at 750°C. Soil available P was extracted with 0.5 M NaHCO 3 . Soil total P and available P were determined by the molybdenum-blue method at a wavelength of 880 nm. Soil available K was extracted with 1 M NH 4 OAc. Soil total K and available K were determined using atomic absorption spectrophotometry. Soil pH was determined in water (soil: water = 1: 2.5). Soil clay, silt and sand were determined by the pipette method. Total fiber content of organic material (cellulose, hemicellulose and lignin) was determined by the method described by van Soest [ 42 ].
Statistical analysis
Statistical analysis of all variables was carried out using the SPSS 16.0 software package. To evaluate the primary factors influencing microbial parameters, we analyzed MBC, MBN, AWCD, H’ , D and U using a two-way analysis of variance (ANOVA) with soil and organic material types as independent factors and permitted to interact. A one-way ANOVA was used to determine the differences of soil properties, chemical characteristics of organic material, and the above microbial parameters among organic material treatments at each soil. The differences among treatments with separation of means by Tukey’s HSD (α = 0.05) test at P < 0.05. Principal component analysis (PCA) of the Ecoplate data was performed to characterize the effect of different organic materials on soil microbial community functions, and the differences of the factor scores of the first principal component (PC1) axis among organic material treatments at each soil were tested using a one-way ANOVA by Tukey’s HSD (α = 0.05) test at P < 0.05. Stepwise multiple regression analysis was applied to determine the key factors influencing microbial properties.
Microbial biomass carbon and nitrogen (MBC and MBN)
At the end of the 1st month, the contributions of soil type and organic material type were significant in explaining the variance in MBC and MBN, and explained 6.9 and 43.6% of the variance in MBC, as well as 9.3 and 50.9% of the variance in MBN, respectively ( P < 0.05; Table 3 ). Significantly higher MBC and MBN were found in Calcaric Cambisol and Luvic Phaeozem than that in Ferralic Cambisol regardless of organic material type ( P < 0.05, Fig 2A and 2C ). When compared with the control, all organic material treatments significantly increased the MBC while only the CM and PM treatments significantly increased the MBN in the three soils ( P < 0.05, Fig 2A and 2C ).
https://doi.org/10.1371/journal.pone.0203812.t003
WS, wheat straw; CS, corn straw; WR, wheat root; CR, corn root; PM, pig manure; CM, cattle manure. Different letters indicate significant differences at P < 0.05 among different materials in the same soil.
https://doi.org/10.1371/journal.pone.0203812.g002
At the end of the 12th month, the variance in MBC and MBN was primarily explained by the organic material type, and the contribution of the organic material type was significant and explained 45.3% of the variance in MBC and 29.5% of the variance in MBN ( P < 0.05, Table 3 ). The WS, CS, WR and CR treatments significantly increased the MBC while only the WS and CS treatments significantly increased the MBN when compared with the control in the three soils ( P < 0.05, Fig 2B and 2D ). When compared with the end of the 1st month, the MBC at the end of the 12th month decreased by 21.5–28.7%, and the MBN at the end of the 12th month increased by 62.9–143.7% in the three soils ( Fig 2 ).
Metabolic activity and microbial functional diversity
At the end of the 1st month, the contributions of soil type and organic material type were significant in explaining the variance in microbial functional diversity ( P < 0.05, Table 3 ). The AWCD and McIntosh index was primarily explained by the organic material species (38.4 and 40.3%, respectively), and the Shannon and Simpson indices were primarily explained by soil type (74.4 and 45.3%, respectively). The microbial functional diversity of Ferralic Cambisol and Luvic Phaeozem in all organic material treatments was significantly increased when compared with the control ( P < 0.05), while only the WS and CS treatments significantly increased all functional diversity indices in Calcaric Cambisol when compared with the control ( P < 0.05, Fig 3A, 3C, 3E and 3G ).
https://doi.org/10.1371/journal.pone.0203812.g003
At the end of the 12th month, the contributions of soil type and organic material type were also significant in explaining the variance in the microbial functional diversity ( P < 0.05, Table 3 ), with 61.8–82.8% of the variances in functional diversity primarily explained by soil type ( P < 0.05, Table 3 ). The WS and WR treatments significantly increased the AWCD, Shannon and McIntosh indices in Ferralic Cambisol and Luvic Phaeozem when compared with the control, and all organic material treatments increased the functional diversity indices in Calcaric Cambisol when compared with the control ( P < 0.05, Fig 3B, 3D, 3F and 3H ).
Carbon substrate utilization patterns of soil microbial communities
To reduce the dimensionality of the data set, a PCA was performed to compare the effect of different organic material treatments on the Biolog Ecoplate utilization patterns of C substrates in the three soils. At the end of the 1st month, the ANOVA for principal component 1 (PC1) indicated that the patterns of substrate utilization between the organic materials and the control treatments were significantly different in Ferralic Cambisol and Luvic Phaeozem ( P < 0.05), and that they were significantly different between the WS and CS treatments and the control in Calcaric Cambisol ( P < 0.05, Fig 4A–4C ). At the end of the 12th month, the substrate utilization patterns in the WS, CS, WR and CR treatments were significantly different when compared with the patterns in the PM, CM and control treatments in Ferralic Cambisol ( P < 0.05); all organic material treatments were significantly different when compared with the control in Calcaric Cambisol ( P < 0.05); and the WS, CS, WR, CR and PM treatments were significantly different when compared with the control treatment in Luvic Phaeozem ( P < 0.05, Fig 4D–4F ).
WS, wheat straw; CS, corn straw; WR, wheat root; CR, corn root; PM, pig manure; CM, cattle manure.
https://doi.org/10.1371/journal.pone.0203812.g004
A high Pearson correlation coefficient (> 0.6) for PC1 in the organic material treatments was shown in Table 4 . At the end of the 1st month, the C substrate use pattern was primarily associated with increased utilization of carbohydrates, amino acids and polymer in Ferralic Cambisol; carbohydrates, amino acids, carboxylic acid, polymer and amine in Calcaric Cambisol; carbohydrates, carboxylic acids, amino acid and polymer in Luvic Phaeozem. At the end of the 12th month, the C substrate use pattern was changed in the three soils. It was associated with increased utilization of carbohydrates, amino acids and amine in Ferralic Cambisol and Calcaric Cambisol, and carbohydrates, amino acids, carboxylic acids and polymer in Luvic Phaeozem.
https://doi.org/10.1371/journal.pone.0203812.t004
The relationships among microbial properties, organic material quality and soil physicochemical properties
The C/N ratio and N content of organic materials significantly affected the MBC and MBN at the end of the 1st and 12th months ( P < 0.05), and soil clay significantly affected MBC at the end of the 1st month ( P < 0.05, Table 5 ). At the end of the 1st month, soil clay content significantly influenced AWCD and U , pH significantly influenced D , and total nitrogen significantly influenced H’ ( P < 0.05). The lignin content of organic materials significantly influenced H’ and D at the end of the 1st month ( P < 0.05). At the end of the 12th month, the soil organic C (SOC) and C/N ratio of organic materials significantly influenced AWCD, H’ , D and U ( P < 0.05), and the clay content significantly influenced H’ and D ( P < 0.05).
https://doi.org/10.1371/journal.pone.0203812.t005
Effects of soil properties and organic materials quality on microbial biomass
Soil microbial biomass represents the amount of microbes in soil, and was successfully used to detect short-term changes in soil functioning to predict organic C accumulation in soil under organic management [ 20 ]. The quality of organic material, e.g., the C availability, the C/N ratio and N content, determines the size of the microbial biomass [ 13 , 43 – 45 ] ( Table 3 ). Carbon sources can provide energy for microorganisms [ 46 – 47 ], and microorganisms can grow rapidly when they encounter abundant C sources, e.g., the significant increase in MBC in organic materials amendment treatments when compared with the control treatment in the three soils at the end of the 1st month ( Fig 2 ). The C/N ratio of organic materials has generally been shown to be a good predictor of the decomposition of organic materials [ 45 , 48 ], and organic materials with low C/N ratio can supply sufficient nutrients for microbes [ 49 – 50 ], which was shown by the significantly higher MBC and MBN in manure treatments than those in crop residue treatment at the end of the 1st month ( Table 2 , Fig 2 ). Nevertheless, at the end of the 12th month ( Fig 2 ) the crop residue amendments with high C/N ratio induced significantly higher MBC and MBN than the manure amendments. Generally, soil N immobilization occurred with organic materials amendment [ 47 , 51 ]. As the experiment proceeded, the amount of available C and N sources decreased and further entered the environment, e.g., as C and N gaseous emissions, dissolved organic C and nitrate leaching under the high precipitation in the experimental subtropical region, especially in the manure amendment treatments; large amounts of easily decomposable and passive decomposable C sources and nutrients were activated by microbial metabolism, and then these activated C sources and nutrients can be easily lost. Conversely, the N limitation was more serious in crop material treatments with high C/N ratio than in manure treatments ( S1 Fig ) [ 46 ]; the immobilized N induced by crop materials can be recycled in microorganisms with crop materials decomposition [ 47 , 52 ]. A 15 N-tracer experiment also demonstrated that organic materials with high C/N ratio prolong nutrient retention in soil through microbial metabolism [ 47 ]. The calculated ratio of MBC to MBN between the 1st and 12th months in this study supports the above phenomenon.
Soil properties had less influence on microbial biomass when compared with the organic material quality, with significant effects only observed at the end of the 1st month ( Table 3 ). In the present study, organic materials amendment might have completely obscured the effect of soil properties on microbial biomass. Generally, soils with high SOC content had high microbial biomass [ 25 – 26 , 53 – 54 ], and nutritional stress might occur when SOC was less than 1% [ 53 ]. When compared with the other two soils in our study ( Table 1 ), Luvic Phaeozem had high microbial biomass because SOC in Luvic Phaeozem is 1.5–2.5 times that of the other two soils. Further, stepwise multiple regression analysis showed that clay content was negatively correlated with MBC after the addition of organic materials at the end of the 1st month. Müller et al. [ 55 ] reported that the clay protective effect of nutrients on microbial biomass was limited, and the increase in clay content could not improve the response of microorganisms to organic material amendment when clay content was > 25%; for example, Ferralic Cambisol had higher clay content than the other two soils in the present study. Meanwhile, the low pH in the Ferralic Cambisol (pH = 5.2) would reduce the utilization of labile substrate by soil microbes [ 56 – 57 ] because of the toxic exchangeable Al in low pH soil [ 58 ]; however, the integrated effect of SOC, clay content and organic materials amendment could affect the response of microbial biomass to pH as shown by the non-significance of pH in explaining microbial biomass in the stepwise multiple regression analysis.
Effects of soil properties and organic materials quality on microbial functional diversity
The average well color development (AWCD) and the functional diversity indices including Shannon, Simpson and McIntosh indices were often used to investigate the general structure and functional potential of soil microbial communities [ 13 , 24 ]. The integrated effect of soil type and organic material amendment significantly ( P < 0.05) affected the microbial functional diversity. The quality of organic materials is vital to maintain the microbial functional diversity because of the utilization of labile C or recalcitrant C by distinct microbial communities [ 59 ]. Lignin is resistant to biodegradation and higher lignin content depresses microbial metabolism; this resulted in the negative correlation between lignin content and the diversity indices (Shannon and Simpson indices) in different organic material treatments at the end of the 1st month [ 17 , 45 ]. At the end of the 12th month in the present study, the microbial functional diversity indices were positively correlated with C/N ratio of organic materials ( Table 5 ), and the microbial communities in crop residue treatments were separated from those in the control treatment in the three soils ( Fig 4 ); this is because the decomposable C sources from crop residue, including cellulose and hemicellulose, and the lower lignin content in crop residues when compared with that in manures supported high microbial functional diversity [ 17 , 44 , 60 ].
Soil properties were more important than organic material properties in explaining the microbial functional diversity as shown in Table 3 [ 13 , 18 – 19 , 24 ]. At the end of the 1st month, the increase in AWCD and McIntosh index with increased clay content was because silt and clay particles generally supported larger and more diverse microbial communities than sand particles [ 61 ]. High soil N content negatively affected soil microbial communities and led to a decrease in the microbial functional diversity by altering the supply and quality of organic matter [ 27 , 62 ]; which resulted in significantly lower Shannon index in Ferralic Cambisol and Luvic Phaeozem than that in Calcaric Cambisol. Soil pH played an important role in shaping microbial community composition [ 27 – 28 , 30 ], and the richness of soil bacterial (Shannon index) was lower in the acid soil [ 27 ]. The present study was not all consistent with the previous reports, although the Shannon index in Ferralic Cambisol and Luvic Phaeozem with lower initial pH ( Table 1 ) was lower than that in Calcaric Cambisol at the end of the 1st month ( Fig 3C ). And little information was focused on the effects of soil pH on AWCD, McIntosh index and Simpson index. The high precipitation in the study site would leach the soluble acid ions into the litter bags, thus limiting organic matter availability and inhibiting microbial metabolism ( Fig 1 ) [ 63 – 64 ]. As a result, AWCD and McIntosh index were low in Calcaric Cambisol because of its high initial pH ( Table 1 ) at the end of the 1st month. As the experiment proceeded, the soil microbial community in Calcaric Cambisol adapted to the experimental environment, and the low nutrient content in Calcaric Cambisol may encourage the microbes to assimilate exogenous C resources from the added organic materials [ 13 , 65 ]; hence, significantly higher microbial functional diversity indices were found in Calcaric Cambisol at the end of the 12th month when compared with those in the 1st month. When compared with the other two soils, Luvic Phaeozem soil had the highest SOC content ( Table 1 ) and significantly lower functional diversity indices at both sampling dates ( Fig 3 ), and the reasons were that (1) Luvic Phaeozem per se had the lowest functional diversity as shown the control treatment ( Fig 3 ), (2) soil with high organic matter has sufficient available C sources for microbial assimilation, and showed reduced assimilation of exogenous C sources by microbes when compared with the Ferralic Cambisol and Calcaric Cambisol with lower organic matter content [ 13 , 65 ]. In addition, soil microbial communities were largely affected by historical factors such as geographic location and soil type due to microbes dwelling in soil [ 20 , 23 , 66 – 67 ]. It has been showed that soil microbial diversity decreased with the increase of latitude and was positively correlated with air temperature [ 68 ], and Luvic Phaeozem in this study was developed from the highest latitude and the annual average temperature (4–5°C) in its local region was lower than the other two soils. Hence, it explained the lower functional diversity in Luvic Phaeozem than the other two soils. Though, Luvic Phaeozem soil transfered from the temperate sub-humid region to the subtropical region, however, the short term effect of climate in this study (≤ 1 year) was not enough to alter the initial microbial communities because it had been reported that no significant responses of climate change on microbial communities within less than 10 years [ 69 ].
Conclusions
Both organic material quality and soil type affected soil microbial characteristics. Organic material quality played a predominant role in controlling the microbial biomass at both sampling periods, and the main parameters of organic matter were C/N ratio and N content. Although manures, with low C/N ratio and high nitrogen content, significantly increased microbial biomass when compared with crop residues at the end of the 1st month ( P < 0.05), the crop residues significantly increased the microbial biomass when compared with manures at the end of the 12th month ( P < 0.05). After the easily available C was exhausted, soil properties regulated the microbial functional diversity, and the main parameters of soil properties were soil organic C and clay content. When compared with the manures, crop residues, in particular straws with low lignin and high C/N ratio, significantly increased the functional diversity indices at both sampling periods ( P < 0.05). This study suggests that the application of straw is a long-term effective measure to increase microbial biomass, and can further induce the changes of soil properties to regulate soil microbial community.
Supporting information
S1 fig. nitrogen remaining in ferralic cambisol, calcaric cambisol, and luvic phaeozem with the amendment of different organic materials..
https://doi.org/10.1371/journal.pone.0203812.s001
S1 Table. Amount of different organic materials in each nylon bag (on the basis of 100:1.5, soil: Added organic carbon ratio).
https://doi.org/10.1371/journal.pone.0203812.s002
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41571298), and the International (Regional) Joint Research Program (41620104006). We thank Catherine Dandie, PhD, from Liwen Bianji, Edanz Group China ( www.liwenbianji.cn/ac ), for editing the English text of a draft of this manuscript.
- View Article
- Google Scholar
- PubMed/NCBI
- 41. Jenkinson DS. The determination of microbial biomass carbon and nitrogen in soil. In Advances in nitrogen cycling in Agricultural Ecosystems (Wilson JR, Ed., CAB international, Wallingford; 1988.
Transect-scale controlling factors for soil microbial biomass carbon
- August 2024
- European Journal of Forest Research

- Nanjing Forestry University

- Forschungszentrum Jülich
- This person is not on ResearchGate, or hasn't claimed this research yet.

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- SOIL BIOL BIOCHEM

- Chuankuan Wang
- Zhenghu Zhou

- Huanchao Zhang

- Paulina B. Ramírez
- Francisco J. Calderón

- Carlos A. Bonilla
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Search Menu
- Sign in through your institution
- Volume 100, Issue 9, September 2024 (In Progress)
- Volume 100, Issue 8, August 2024 (In Progress)
- Volume 100, Issue 7, July 2024 (In Progress)
- Volume 100, Issue 6, June 2024
- Advance articles
- Editor's Choice
- Awards & Prizes
- Thematic Issues
- Virtual Special Issues
- FEMS Journals
- FEMS Microbiology Letters
- FEMS Microbiology Reviews
- FEMS Yeast Research
- Pathogens and Disease
- FEMS Microbes
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- About FEMS Microbiology Ecology
- About the Federation of European Microbiological Societies
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, standardisation in soil microbiology: dealing with the natural complexity and diversity, current standards in soil microbiology, directions for future standards, the iso standardisation process, fictitious, cultural and real hurdles, final remarks, acknowledgements, standardisation of methods in soil microbiology: progress and challenges.
Editor: Lily Young
- Article contents
- Figures & tables
- Supplementary Data
Laurent Philippot, Karl Ritz, Pascal Pandard, Sara Hallin, Fabrice Martin-Laurent, Standardisation of methods in soil microbiology: progress and challenges, FEMS Microbiology Ecology , Volume 82, Issue 1, October 2012, Pages 1–10, https://doi.org/10.1111/j.1574-6941.2012.01436.x
- Permissions Icon Permissions
A plethora of methods have been developed over the few last decades to enable a better understanding of the ecology of soil microbial communities and their roles in soil functioning. However, there is generally considerable variation (both subtle and more extensive) in the actual realisation of these methods, and limited efforts have been devoted to their standardisation, despite this being crucial to underpin data comparison and integration. Ensuring comparable data across studies through standardisation is arguably best-practice, as well as necessary to effectively meet the objectives of various schemas, which require assessment of the consequences of the global change and intensification of human activities on the functioning of the soil ecosystem and its biological diversity. This article presents an overview of the existing and forthcoming ISO standards in soil microbiology and highlights possible future research efforts to be undertaken for developing new standards. We also discuss some practical and theoretical bottlenecks and hurdles that have limited standardisation in soil microbiology up to now.
Microorganisms in soil ecosystems are ubiquitous, abundant, diverse and essential for many soil functions such as carbon and nitrogen cycling, plant productivity and climate regulation (Whitman et al ., 1998 ; Torsvik et al ., 2002 ; Falkowski et al ., 2008 ; van der Heijden et al ., 2008 ; Bodelier, 2011 ). Because of their importance, there is a large volume of past and contemporary researches that aims to understand the ecology of soil microbial communities, with thousands of articles devoted to this research field published annually. Numerous methods have been developed to estimate abundance, diversity and activity of soil microorganisms. Several such procedures are now successfully applied on a regular and on-going basis, perhaps most notably the chloroform fumigation-extraction technique for estimating microbial biomass (Vance et al ., 1987 ), and DNA fingerprinting approaches for estimating the structure of microbial communities. Perversely, many of these methods become victims of their own success, and a plethora of laboratory- or even user-specific protocols, which contain minor to major modifications of the initially described methods, are now used worldwide. However, these differences between protocols are far from being inconsequential as they often include inherent bias, which hamper data comparison across studies, let alone laboratories. Indeed, variations in data obtained by different laboratories or using different protocols are commonly reported (Ocio & Brookes, 1990 ; Beck et al ., 1997 ; Krsek & Wellington, 1999 ; Martin-Laurent et al ., 2001 ; Creamer et al ., 2009 ; Pan et al ., 2010 ). A theoretically obvious, albeit practically challenging, solution is to define and use standardised methods. This is becoming all the more important because an exponentially increasing volume of data is now being generated, particularly with the advent of automated or high-throughput techniques, notably in relation to molecular biology. Such techniques offer exciting opportunities for better understanding soil microbial diversity, how it relates to soil functions, and more effective ways to manage terrestrial ecosystems to meet the challenges of sustainability. This grand challenge should be facilitated by ensuring comparable data, which is necessary in order that our knowledge of soil microbial communities can be effectively integrated.
The concept, and practice, of standardisation in soil microbiological assays can be applied at a range of levels, from the individual researcher/group (vital to ensure coherence within a body of experimentation), through institutional (assists integration and coherence within institutional-level programmes), to national (e.g. British Standards and French National Organisation for Standardisation) and international [e.g. International Organisation for Standardisation, (ISO)]. Here we focus on the latter context, as this is arguably the most effective route to achieve the higher-level aims of standardisation. Moreover, science itself is an international collaborative effort and comparisons across studies need to be performed beyond country borders, not least because soils and the organisms they support operate entirely independently of such boundaries. Standards providing internationally agreed methods for assessing soil microorganisms have mostly been developed by the International Organisation for Standardisation (ISO). However, the number of ISO standardised methods is still scant in relation to the numerous methods that have been developed within the field of soil microbiology. In addition, the use of ISO methods in soil microbiology research articles, outside of ecotoxicology studies, is in our perception relatively rare. In this article, we underline the importance of standardisation in soil microbiology, present an overview of the existing and forthcoming ISO standards, and discuss some technical and cultural hurdles. One aim is to stimulate debate in this field and to encourage a move toward the development and greater dissemination of internationally agreed standards in soil microbiology.
Soils are arguably the most complex systems on the planet, given the extraordinary diversity of their chemical and biological constituents, as well as the extreme structural heterogeneity (Ritz, 2008 ). There are also a wide range of soil types, with huge numbers of classes of soil recognised in taxonomic schemes both at global down to national scales, for example, some 748 Soil Series are recognised in the Soil Survey of England and Wales (Clayden & Hollis, 1984 ) and thousands of types in the lower-order taxa of World Reference Base (FAO, 2006 ). The geo-spatial distribution of soils is also complex across virtually all size scales, which means that studies at almost any spatial scale involve a variety of soil types, which may confound the ready application of standard techniques. This diversity of constitution and basic characteristics severely challenges the ability to set standards in measuring soil properties and processes. This is particularly true for biological aspects of soil systems, and in part accounts for the concomitant diversity in methodological variants. Even something as outwardly straightforward as determining soil organic carbon is confounded by the fact that soils can vary from essentially 0–100% organic matter, there is potential (and variable) interference from inorganic forms of carbon, and the same procedure is certainly not appropriate for soils at the two extremes (Nelson & Sommers, 1996 ). It is often then the case that no single method is universally appropriate and that variants within methods are needed to compensate for differences in properties that may occur if they are to be applicable to the gamut of soils. For example, measuring soil respiration by CO 2 emission is relatively straightforward if the pH of the soil is lower than 7.5, but in more alkaline soils, the partition coefficient of CO 2 between air and water starts to confound the technique because proportionately more CO 2 will prevail in the pore water (Anderson, 1982 ). The quality and quantity of organic matter and clay vary between soils that affects the nature and extent of potential absorption of biochemicals, notably nucleic acids, such that a range of devices to counter such effects need to be applied, contingent on the soil. These factors can be compensated for by variants in technique, and such variants can be duly standardised. In principle, such matters do not then preclude the setting of standards, but they certainly prevent the setting of simple standards. Furthermore, there is a significant issue that affects data comparability, as with complex protocols, there is an increased likelihood that different operators will determine different absolute values for measurements, because of accumulations of even subtle differences between each of the steps in such procedures.
Another factor arising from the need for sophisticated/adjusted/complex protocols is the ease with such protocols are agreed upon within the context of a standards setting framework, particularly an international one. This is because the optimal procedures are not necessarily readily defined and can become more a matter of best judgement. For example, it can be argued either way that the pH of the buffer medium in enzyme assays should be standardised to a particular pH, or the pH of the particular soil under scrutiny (German et al ., 2011 ), but there are then supplementary issues of how to determine that pH. Another concern is at which temperature one should measure soil respiration? The same for a sub-arctic tundra soil as one from Namibia or a ‘locally pertinent’ temperature? And then what moisture content is optimal for respiration measurements and how should that be determined? Such questions are undoubtedly very important in defining standards but challenge the attainment of scientific consensus.
Despite the inherent complexity and diversity of soils described earlier, some methods to study soil microorganisms have been standardised since 1997 (Table 1 ). Due to a strong concern regarding the degradation of soils in relation to local and diffuse contamination or loss of biodiversity, the existing standards were developed by the ‘Soil quality’ Technical Committee ISO/TC 190 with a strong focus on assessing the effects of chemicals and pollution on the soil fauna and soil microorganisms (Nortcliff, 2002 ). Methods for measuring soil microbial biomass using substrate-induced respiration and fumigation-extraction were the first ones to be standardised in the field of soil microbiology in the late nineties (ISO 14240, Table 1 ). Indeed, these methods based on pioneering work of Vance et al . ( 1987 ) were proposed to provide a sensitive indicator for measuring changes in the total quantity of soil microorganisms in response to environmental factors or anthropogenic disturbances. Most of the other existing ISO standards were developed for similar purposes and are therefore biased toward effective monitoring of the soil microbial community to meet extant policy requirements (Table 1 ). This trend is particularly obvious for ISO 14238 ‘Determination of nitrogen mineralisation and nitrification in soils and the influence of chemical on these processes’ and ISO 15473 ‘Testing for biodegradation of organic chemicals in soil’. Thus, ISO 14238 was designed to determine the effects of different concentrations of a chemical on the N-cycling processes using dose–response curves while ISO 15473 gives general guidelines for the selection and method of tests to determine the biological degradation of organic chemicals introduced into the soil either intentionally or accidentally.
ISO standardised methods in soil microbiology
| Year | Method | ISO reference | Bibliography |
| 1997 | Determination of soil microbial biomass – part 1: substrate-induced respiration method | ISO 14240-1 | Jenkinson & Powlson ( ); Anderson & Domsch ( ) |
| 1997 | Determination of soil microbial biomass – part 2: fumigation-extraction method | ISO 14240-2 | Brookes . ( ); Vance . ( ); Ocio & Brookes ( ); Sparling . ( ); Wu . ( ); Inubushi . ( ); Mueller . ( ); Harden . ( , ) |
| 1997 | Determination of nitrogen mineralization and nitrification in soils and the influence of chemicals on these processes | ISO 14238 | Bremner ( ); Henriksen & Selmer-Olsen ( ); Selmer-Olsen ( ); Stanford & Smith ( ); Andersch & Anderson ( ) |
| 2002 | Determination of abundance and activity of soil microflora using respiration curves | ISO 17155 | Anderson & Domsch ( ); Nordgren . ( ); Arnebrant & Schnurer ( ); Chander & Brookes ( ); VanBeelen . ( ); Stenstrom . ( ); Wilke . ( ) |
| 2002 | Soil quality – guidance on laboratory testing for biodegradation of organic chemicals in soil under anaerobic conditions | ISO 15473 | Beland . ( ); Gowda & Sethunathan ( ); Healy & Young ( ); Attaway . ( ); Kearney ( ); Shelton & Tiedje ( ); Ward ( ); Alef & Nannipieri ( ) |
| 2002 | Laboratory methods for determination of microbial soil respiration | ISO 16072 | Gupta & Singh ( ); Nordgren ( ); Watts . ( ) |
| 2004 | Determination of potential nitrification and inhibition of nitrification – rapid test by ammonium oxidation | ISO 15685 | Belser & Mays ( ); Hansson . ( ); Stenberg . ( ); Winkel . ( ) |
| 2005 | Determination of dehydrogenase activity in soils – part 1: method using triphenyltetrazolium chloride (TTC) | ISO 23753-1 | Thalmann ( ); Glathe & Thalmann ( ); Wilke ( ); Ohlinger ( ) |
| 2005 | Determination of dehydrogenase activity in soils – part 2: method using iodotetrazolium chloride (INT) | ISO 23753-2 | Thalmann ( ); Glathe & Thalmann ( ); vonMersi & Schinner ( ); Spothelfer-Magaña . ( ); Fuchs . ( ); Ohlinger ( ) |
| 2010 | Measurement of enzyme activity patterns in soil samples using fluorogenic substrates in micro-well plates | ISO 22939 | Tabatabai ( ); Stemmer . ( ); Marx . ( ); Vepsäläinen . ( , ); Marx . ( ); Niemi & Vepsalainen ( ) |
| 2010 | Determination of soil microbial diversity – part 1: method by PLFA analysis and PLEL analysis | ISO 29843-1 | Blight & Dyer ( ); White . ( ); Findlay . ( ); Frostegård . ( ); Zelles & Bai ( ); Alef & Nannipieri ( ); Zelles ( ); Gattinger . ( ) |
| 2011 | Determination of soil microbial diversity – part 2: method by PLFA analysis using the ‘simple PLFA extraction method’ | ISO 29843-2 | Blight & Dyer ( ); White . ( ); Zelles & Bai ( ); Gattinger . ( ) |
| 2011 | Method to directly extract DNA from soil samples | ISO 11063 | Tsai & Olson ( ); Smalla . ( ); Zhou . ( ); van Elsas . ( ); Martin-Laurent . ( ); Niemi . ( ) |
| Year | Method | ISO reference | Bibliography |
| 1997 | Determination of soil microbial biomass – part 1: substrate-induced respiration method | ISO 14240-1 | Jenkinson & Powlson ( ); Anderson & Domsch ( ) |
| 1997 | Determination of soil microbial biomass – part 2: fumigation-extraction method | ISO 14240-2 | Brookes . ( ); Vance . ( ); Ocio & Brookes ( ); Sparling . ( ); Wu . ( ); Inubushi . ( ); Mueller . ( ); Harden . ( , ) |
| 1997 | Determination of nitrogen mineralization and nitrification in soils and the influence of chemicals on these processes | ISO 14238 | Bremner ( ); Henriksen & Selmer-Olsen ( ); Selmer-Olsen ( ); Stanford & Smith ( ); Andersch & Anderson ( ) |
| 2002 | Determination of abundance and activity of soil microflora using respiration curves | ISO 17155 | Anderson & Domsch ( ); Nordgren . ( ); Arnebrant & Schnurer ( ); Chander & Brookes ( ); VanBeelen . ( ); Stenstrom . ( ); Wilke . ( ) |
| 2002 | Soil quality – guidance on laboratory testing for biodegradation of organic chemicals in soil under anaerobic conditions | ISO 15473 | Beland . ( ); Gowda & Sethunathan ( ); Healy & Young ( ); Attaway . ( ); Kearney ( ); Shelton & Tiedje ( ); Ward ( ); Alef & Nannipieri ( ) |
| 2002 | Laboratory methods for determination of microbial soil respiration | ISO 16072 | Gupta & Singh ( ); Nordgren ( ); Watts . ( ) |
| 2004 | Determination of potential nitrification and inhibition of nitrification – rapid test by ammonium oxidation | ISO 15685 | Belser & Mays ( ); Hansson . ( ); Stenberg . ( ); Winkel . ( ) |
| 2005 | Determination of dehydrogenase activity in soils – part 1: method using triphenyltetrazolium chloride (TTC) | ISO 23753-1 | Thalmann ( ); Glathe & Thalmann ( ); Wilke ( ); Ohlinger ( ) |
| 2005 | Determination of dehydrogenase activity in soils – part 2: method using iodotetrazolium chloride (INT) | ISO 23753-2 | Thalmann ( ); Glathe & Thalmann ( ); vonMersi & Schinner ( ); Spothelfer-Magaña . ( ); Fuchs . ( ); Ohlinger ( ) |
| 2010 | Measurement of enzyme activity patterns in soil samples using fluorogenic substrates in micro-well plates | ISO 22939 | Tabatabai ( ); Stemmer . ( ); Marx . ( ); Vepsäläinen . ( , ); Marx . ( ); Niemi & Vepsalainen ( ) |
| 2010 | Determination of soil microbial diversity – part 1: method by PLFA analysis and PLEL analysis | ISO 29843-1 | Blight & Dyer ( ); White . ( ); Findlay . ( ); Frostegård . ( ); Zelles & Bai ( ); Alef & Nannipieri ( ); Zelles ( ); Gattinger . ( ) |
| 2011 | Determination of soil microbial diversity – part 2: method by PLFA analysis using the ‘simple PLFA extraction method’ | ISO 29843-2 | Blight & Dyer ( ); White . ( ); Zelles & Bai ( ); Gattinger . ( ) |
| 2011 | Method to directly extract DNA from soil samples | ISO 11063 | Tsai & Olson ( ); Smalla . ( ); Zhou . ( ); van Elsas . ( ); Martin-Laurent . ( ); Niemi . ( ) |
UR, under revision; UP, under publication.
Criteria related to applicability and effectiveness of standards for routine analyses such as high throughput analysis, cost, usability or data interpretation have up to now excluded molecular methods, such as terminal fragment length polymorphism for assessing microbial diversity, despite their widespread use in research. However, among the new ISO standards, the development of the ISO 11063 standard for soil DNA extraction (Petric et al ., 2011 ) is of special interest because it is the first step of all PCR-, hybridisation, and sequencing-based molecular analyses of the diversity and abundance of soil microbial communities. As a result, thousands of studies are performed yearly in environmental microbiology using soil DNA extraction methods. Due to this important business market, at least ten companies are commercialising soil DNA extraction kits, which add to the list of home-made protocol. This is despite it being well established that the apparent microbial diversity determined by any nucleic acid analysis procedure is contingent on the DNA extraction method (Frostegård et al ., 1999 ; Martin-Laurent et al ., 2001 ; deLiphtay et al ., 2004 ; Feinstein et al ., 2009 ; Pan et al ., 2010 ; Delmont et al ., 2011 ). The ISO 11063 standard for soil DNA extraction is based on both chemical and physical approaches for extraction and lyses of the microbial cells as described by Petric et al . ( 2011 ). This ISO is timely since studies of soil microbial diversity based on soil DNA extraction are generating an exponential amount of sequence data, and large scale projects aiming at sequencing the soil metagenome are now launched (Vogel et al ., 2009 ). Knowledge of the identity and the quantity of each compound used in the ISO 11063 or any ISO protocol provides transparency and allow users a complete quality control, which is a major advantage over commercial kits. Thus, production batch effects can occur, and this has been observed for some commercial soil DNA extraction kits (unpublished data). A transparent protocol also avoids the risk of subsequent modifications of the kit reagents by companies or risks associated to the versatility of their business strategies such acquisition and merging, which are common activities for biotechnology industry.
While no nucleic acid-based method for assessing soil microbial diversity have yet been proposed for international standardisation, two lipid-based methods have recently became ISO standards (Table 1 ). Phospholipid fatty acid (PLFA) and phospholipid ether lipids (PLEL) analyses are rapid and inexpensive methods for providing a quantitative measure of the viable soil biomass and complex microbial community profiles. They offer the advantage of targeting the entire microbial community, thus allowing calculation of the fungal/bacteria ratio using markers PLFA specific of these domains (Frostegård & Bååth, 1996 ). Since the late 1990s, several comprehensive reviews discussing the strengths and weaknesses of the use of lipid fatty acids for assessing microbial biomass and community structure in soil have been published (Olsson, 1999 ; Zelles, 1999 ; Kaur et al ., 2005 ; Frostegård et al ., 2011 ). Unfortunately, while some of the ISO standards described in Table 1 have been published more than 10 years ago, their use by the scientific community is still very limited. Thus, the ISO has no power to enforce the implementation of the standards it develops and therefore adoption of the ISO standard is still mainly voluntary.
The standardisation effort is uneven between methods addressing the abundance, the diversity and the activity of the soil microbial community. Indeed, while there are already three ISO standards for quantifying soil microbial biomass, a new work item proposing a standard to estimate the abundance of the soil bacterial community by 16S rRNA gene targeted quantitative PCR (qPCR) was recently adopted by the Soil quality ISO technical committee (Australia, September 2011). The recent developments of qPCR analyses also allow the quantification of the abundances of specific functional or taxonomical microbial groups, which may represent useful bioindicators (Wessen & Hallin, 2011 ). With the use of appropriate blanks, internal and surrogate standards, qPCR is a reliable method having the advantage to offer high throughput and cost-effective analyses.
For a better understanding of soil microbial activity, or more generally of soil functioning, several methods for quantifying potential enzyme activity have been developed. Even though these methods providing an insight of the size of the enzyme pool have some limits (Wallenstein & Weintraub, 2008 ), they are commonly used as microbiological indicators of soil quality and should therefore be standardised for comparison of microbial activities both between soils and laboratories. For example, because of their environmental and agronomical importance, microorganisms involved in N-cycling are of key interest. In addition, they are popular models in soil microbial ecology for relating microbial diversity and soil functioning. However, only measurement of potential nitrification has been internationally standardised up to now, while methods for monitoring other N-processes such as nitrogen fixation and denitrification also necessitate standardisation. For example, the original protocol for estimating potential denitrification (Smith & Tiedje, 1979 ) has been modified in many ways. In this assay, to measure the activity of the pool of denitrification enzymes in the soil at the time of sampling, soil slurries are incubated in the laboratory in non-limiting denitrification conditions (without oxygen, addition of nitrate and carbon, and of chloramphenicol to avoid de novo synthesis) so that only the amount of enzyme is rate-limiting. Changes in the original protocol include excluding the chloramphenicol, which can decrease the activity of synthesized enzymes, addition of different carbon types and amount (glucose, acetate, glumatic acid, etc) and incubation of the soil slurries in various conditions. Similarly, determination of the nitrogenase activity using the acetylene reduction technique (Hardy et al ., 1968 ) is subjected to various modifications of the protocol resulting, for example, in variants of the acetylene concentration (0.03–0.1 v/v). In contrast to other methods, most modifications of these methods are not soil-specific and both potential denitrification and nitrogen-fixation assays could readily be standardised in future.
Finally, regarding methods to monitor the diversity and the structure of the soil microbial community, the adoption of the ISO 29843 for PLFA and PLEL analyses opens the path for other standards. While it is too early to propose any standardisation of the new high-throughput sequencing technologies (e.g. 454 pyrosequencing, etc…), other powerful approaches such as those based on taxonomic and functional microarrays meet the criteria to become standards. Of course these perspectives for the development of future standards in soil microbiology are not exhaustive, and we encourage soil microbiologists to expand it by proposing other popular methods for standardisation.
If one is interested in developing new international standards, it is worth reviewing how standards are developed within the ISO framework. According to ISO, a standard is a document that is established by consensus and approved by a recognised body (ISO/IEC, 2004 ). It provides, for common and repeated use, rules, guidelines or characteristics for activities or their results, aimed at the achievement of the optimum degree of order in a given context. Standards should be based on the consolidated results of science, technology and experience, and aimed at the promotion of optimum community benefits (ISO/IEC, 2004 ). Different types of standards can be developed within this framework (e.g. terminology, product, process, service, testing standards). Such standards are elaborated by technical committees and/or subcommittees that usually comprise representatives from the industrial, technical, business sectors as well as representatives of government agencies, testing laboratories, consumer associations, non-governmental organizations and academia.
The standardisation process includes six successive stages, taking place over a time period usually not exceeding 48 months: viz . proposal, preparatory, committee, enquiry, approval and publication stages (ISO/IEC, 2009 ) (Fig. 1 ). To confirm the need for the development of a new standard, the new work item proposal should be supported by scientific papers presenting the scientific background, and some results demonstrating the applicability and the relevance of the method. A proposal is accepted when at least five participating countries vote positively and nominate experts to participate actively in its development. The first draft of the method is submitted to the experts for discussion and improvements until a consensus has been reached on the technical content. Then, the draft document is distributed for voting and comments by the participating countries of the technical or sub-committees. In case of major disagreements, successive committee drafts may be considered before submission of the text as a draft international standard.

Flow chart summarising the different steps for standardising a new test method within the ISO framework.
The validation process of a future standard is crucial before publication as an international standard. It involves laboratories from National Bodies of the relevant technical or sub-committees (but not exclusively) for evaluation of the reproducibility of the test method under standardisation. The resulting performance characteristics of this inter-laboratory trial are part of the standard. When all due processes have been satisfactorily completed, the standard is then officially published and released for adoption. International standards are then reviewed at the least 3 years after publication and every 5 years after the first review by all the ISO member bodies to incorporate, in particular, improvements of the method or technical changes. During this review process, members of the technical or sub-committees decide whether the standard should be confirmed, revised or withdrawn.
As underlined by Pan et al . ( 2010 ), inter-calibration of protocols is not a common practice in environmental microbiology. As a consequence, while an impressive list of methods, regularly summarised in books, has been developed for studying microorganisms in soils, limited effort has been devoted to standardisation. This paradox is accentuated by the fact that most of these methods are subjected to almost endless modifications of their protocols, which can affect the results and hamper data comparison. These subtle to deep changes can be as a result of weaknesses in the original protocols, which are often related to a failure when applied to a different soil. However, a large number of variations in protocols can still be found in the literature for similar or even identical soils. One could therefore ask whether the existence of so many deviating protocols only reflects a true need for modifications because of the overwhelming diversity and complexity of the soils, or if there are other factors involved conveyed by a certain lack of rigor.
Possibly the fact that soil microbiology is still facing an tremendous and ongoing method development can be considered as contradictory to developing standards. However, evolving fields with technological evolution, new methods or new quality and safety requirements are not an obstacle to standardisation. Indeed, in biomedical science, laboratory-based medical and scientific microbiologists from throughout the Health Protection Agency in Scotland have developed the National Standards Methods, which include, for example, a standard for the detection of influenza viruses by qPCR. Within the ISO, all existing standards are reviewed at intervals of not more than 5 years to evaluate whether a revision is required. This is, for example, the case of the ISO 15685 ‘Determination of potential nitrification and inhibition of nitrification – rapid test by ammonium oxidation’, which was revised in 2011.
Another obstacle could be the naïve thinking that certain of our methods are inadequate for standardisation. It is essential that standardised methods provide meaningful information, but not that they are ‘perfect’. In soil microbiology, such perfection would apply to an assay that provides a true picture of microorganisms’ activity, diversity or abundance in the soil. Given the complexity of the soil system and inherent biodiversity, this may in any case be untenable. As the accuracy of any method in soil microbiology cannot be estimated directly but only through the prism of other methods, microbiologists are facing a potentially unsolvable paradox. In addition, sample-specific optimisation of methods can lead to ‘nearsightedness’, the more detailed description of the studied soil being at the price of not seeing the bigger picture because of the impossibility to compare and integrate data across studies.
Evaluation of the best protocol to standardise is also often hampered by a trade-off situation in which one advantage is lost for another. An example of such a circumstance is the trade-off in relation to soil DNA extraction where the DNA yield can be increased, but typically at the cost of lower quality which may then compromise its apparent representativity, particularly where annealing processes are important.
In the recent years, increasing efforts have been made to promote consistency among laboratories. These efforts were mostly devoted to improving standardisation and transparency in metadata capture and exchange such as the minimum information about a genome sequence (Field et al ., 2008 ), the minimum information about a marker gene sequence (Yilmaz et al ., 2010 ) or the genomic standards consortium: bringing standards to life for microbial ecology (Yimaz et al ., 2011 ). As protocols continue to evolve and diversify, guidance modules for reporting in a standardised manner, the use of techniques have also been described. Thus, the lack of consensus on how to perform qPCR experiments has led Bustin et al . ( 2009 ) to propose the minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines. There are several precedents such as the minimum information about a proteomics experiment (Taylor et al ., 2007 ) or the minimum information about a microarray experiment (MIAME) (Brazma et al ., 2001 ). The MIAME is now an accepted reference as the reflected by the number of citations, which exceed 1600 (ISI Web of Knowledge). These efforts also highlight that there are other paths for standardisation than the ISO. However, standardisation should proceed within the auspices of international working bodies and be preferably in open access or with a very low cost to facilitate the dissemination within the scientific community. Standard adoption also requires both information and a stronger involvement of leading researchers within the field. There is a clearly a need and room for new standards in soil microbiology. New standards would be beneficial to researchers, non-governmental organisations, governments, farmers and other land managers, for better monitoring soil quality and understanding of soil functioning. Developing standard protocols in soil microbiology is crucial to meet the objectives of the Millennium Ecosystem Assessment ( 2005 ) and of the emerging EU Soil Framework Directive (Commission of the European Community, 2006 ) for assessing the consequences of the intensification of human activities on the functioning of the soil ecosystem and its biodiversity.
In conclusion, we argue that there is a need to avoid the perhaps inevitable procrastination in setting standards that arises from the range of issues discussed earlier, and we need to be pragmatic in getting standards accepted and implemented, with caveats duly acknowledged. There is a trade-off between the urge for perfect methods vs. standardised methods, and we believe that standardisation allowing data comparison across studies, and therefore facilitating the quest for ‘unifying principles in soil ecology’ as described by Fierer et al . ( 2009 ), is more important than describing a few specific samples ‘perfectly’. The rewards from such an approach would far exceed the drawbacks.
We would like to thank many colleagues who have, directly or indirectly, contributed to the ideas presented in this work. This work was partly supported by the European Commission within EcoFINDERS project (FP7-264465) and the Ecofun Microbiodiv project (FP7 ERA NET 216/01).
Alef K Nannipieri P ( 1995 ) Methods in Applied Soil Microbiology and Biochemistry . Academic Press , London, UK .
Google Scholar
Google Preview
Andersch I Anderson JPE ( 1991 ) Influence of pesticides on nitrogen transformations in soil . Toxicol Environ Chem 30 : 153 – 158 .
Anderson JPE ( 1982 ) Soil respiration . Methods of Soil Analysis, Part 2 , 2nd edn ( Page A Miller RH Keeney DR , eds), pp. 837 – 871 . American Society of Agronomy and Soil Science Society of America , Madison, WI, USA .
Anderson JPE Domsch KH ( 1978 ) Physiological method for quantitative measurement of microbial biomass in soils . Soil Biol Biochem 10 : 215 – 221 .
Arnebrant K Schnurer J ( 1990 ) Changes in ATP content during and after chloroform fumigation . Soil Biol Biochem 22 : 875 – 877 .
Attaway HH Paynter MJB Camper ND ( 1982 ) Degradation of selected phenylurea herbicides by anaerobic pond sediment . J Environ Sci Health B 17 : 683 – 699 .
Beck T Joergensen RG Kandeler E Makeschin F Nuss E Oberholzer H Scheu S ( 1997 ) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C . Soil Biol Biochem 29 : 1023 – 1032 .
Beland FA Farwell SO Geer RD ( 1974 ) Anaerobic degradation of 1,1,1,2-tetrachloro-2,2-bis(P-chlorophenyl)ethane(DTE) . J Agric Food Chem 22 : 1148 – 1149 .
Belser L Mays E ( 1980 ) Specific inhibtion of nitrite oxidation by chlorate and its use in assessing nitrification in soil and sediment . Appl Environ Microbiol 39 : 505 – 510 .
Blight EG Dyer WJ ( 1959 ) A rapid method of total lipid extraction and purification . Can J Biochem Physiol 37 : 911 – 917 .
Bodelier P ( 2011 ) Towards understanding, managing and protecting microbial ecosystems . Front Microbiol 2 : 1 – 8 .
Brazma A Hingamp P Quackenbush J et al. ( 2001 ) Minimum information about a microarray experiment (MIAME) – toward standards for microarray data . Nat Genet 29 : 365 – 371 .
Bremner JM ( 1965 ) Nitrogen availability indexes . Methods of Soil Analysis, Part 2 ( Black CA , ed), pp. 1324 – 1345 . American Society of Agronomy , Madison, WI .
Brookes PC Landman A Pruden G Jenkinson DS ( 1985 ) Chloroform fumigation and the release of soil-nitrogen - a rapid direct extraction method to measure microbial biomass nitrogen in soil . Soil Biol Biochem 17 : 837 – 842 .
Bustin SA Benes V Garson JA et al. ( 2009 ) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments . Clin Chem 55 : 611 – 622 .
Chander K Brookes PC ( 1991 ) Effects of heavy-metals from past applications of sewage-sludge on microbial biomass and organic-matter accumulation in a sandy loam and silty loam UK soil . Soil Biol Biochem 23 : 927 – 932 .
Clayden B Hollis J ( 1984 ) Critieria for differentiating soil series . Soil Survey Technical Monograph , Vol. 17. Rothamsted Experimental Station , Harpenden, UK , pp. 159.
Commission of the European Community ( 2006 ) Directive of the European parlament and of the council establishing a framework for the protection of soil and amending directive 2004/35/EC .
Creamer RE Bellamy P Black HIJ et al. ( 2009 ) An inter-laboratory comparison of multi-enzyme and multiple substrate-induced respiration assays to assess method consistency in soil monitoring . Biol Fertil Soil 45 : 623 – 633 .
Delmont TO Robe P Cecillon S Clark IM Constancias F Simonet P Hirsch PR Vogel TM ( 2011 ) Accessing the soil metagenome for studies of microbial diversity . Appl Environ Microbiol 77 : 1315 – 1324 .
Falkowski PG Fenchel T Delong EF ( 2008 ) The microbial engines that drive Earth's biogeochemical cycles . Science 320 : 1034 – 1039 .
FAO ( 2006 ) World Reference Base for Soil Resources Reports No. 130 . FAO , Rome .
Feinstein LM Sul WJ Blackwood CB ( 2009 ) Assessment of bias associated with incomplete extraction of microbial DNA from soil . Appl Environ Microbiol 75 : 5428 – 5433 .
Field D Garrity G Gray T et al. ( 2008 ) The minimum information about a genome sequence (MIGS) specification . Nat Biotechnol 26 : 541 – 547 .
Fierer N Grandy A Six J Paul E ( 2009 ) Searching for unifying principles in soil ecology . Soil Biol Biochem 41 : 2249 – 2256 .
Findlay RH Trexler MB Guckert JB White DC ( 1990 ) Laboratory study of disturbance in marine-sediments – response of a microbial community . Mar Ecol Prog Ser 62 : 121 – 133 .
Frostegård Å Bååth E ( 1996 ) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil . Biol Fertil Soil 22 : 59 – 65 .
Frostegård Å Tunlid A Bååth E ( 1991 ) Microbial biomass measured as total lipid phosphate in soils of different organic content . J Microbiol Methods 14 : 151 – 163 .
Frostegård Å Courtois S Ramisse V et al. ( 1999 ) Quantification of bias related to the extraction of DNA directly from soils . Appl Environ Microbiol 65 : 5409 – 5420 .
Frostegård Å Tunlid A Bååth E ( 2011 ) Use and misuse of PLFA measurements in soils . Soil Biol Biochem 43 : 1621 – 1625 .
Fuchs M Koch C Wilke BM ( 1994 ) Modification of the determination of dehydrogenase activity with tetrazolium chloride for heavy metal contaminated soils) . VDLUFA Schriftenreihe 38 : 899 – 902 .
Gattinger A Günthner A Schloter M Munch J ( 2003 ) Characterization of Archaea in soils by polar lipid analysis . Acta Biotechnol 23 : 21 – 28 .
German DP Weintraub MN Grandy AS Lauber CL Rinkes ZL Allison SD ( 2011 ) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies . Soil Biol Biochem 43 : 1387 – 1397 .
Glathe H Thalmann A ( 1970 ) The microbial activity and its relationship to fertility characteristics of various farm lands with special reference to the dehydrogenase activity (TTC reduction). 2. Determination of the TTC reduction in soil in laboratory trials . Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 124 : 24 – 36 .
Gowda TKS Sethunathan N ( 1976 ) Persistence of endrin in Indian rice soils under flooded conditions . J Agric Food Chem 24 : 750 – 753 .
Gupta SR Singh JS ( 1977 ) Effect of alkali concentration, volume and absorption area on measurement of soil respiration in a tropical sward . Pedobiologia 17 : 233 – 239 .
Hansson G-B Klemedtsson L Stenström J Torstensson L ( 1991 ) Testing the influence of chemicals on soil autotrophic ammonium oxidation . Environ Toxicol Water Qual 6 : 351 – 360 .
Harden T Joergensen RG Meyer B Wolters V ( 1993a ) Mineralization of straw and formation of soil microbial biomass in a soil treated with simazine and dinoterb . Soil Biol Biochem 25 : 1273 – 1276 .
Harden T Joergensen RG Meyer B Wolters V ( 1993b ) Soil microbial biomass estimated by fumigation extraction and substrate-induced respiration in 2 pesticide-treated soils . Soil Biol Biochem 25 : 679 – 683 .
Hardy R Holsten R Jackson E Burns R ( 1968 ) The acetylene-ethylene assay for N 2 fixation: laboratory and field evaluation . Plant Physiol 43 : 1185 – 1207 .
Healy JB Young LY ( 1979 ) Anaerobic biodegradation of 11 aromatic-compounds to methane . Appl Environ Microbiol 38 : 84 – 89 .
Henriksen A Selmer-Olsen AR ( 1970 ) Automatic methods for determining nitrate and nitrite in water and soil extracts . Analyst 95 : 514 – 518 .
Inubushi K Brookes PC Jenkinson DS ( 1991 ) Soil microbial biomass C, N and ninhydrin-N in aerobic and anaerobic soils measured by the fumigation-extraction method . Soil Biol Biochem 23 : 737 – 741 .
ISO/IEC ( 2004 ) Guide 2 – Standardization and Related Activities – General Vocabulary . International Organization for Standardization , Geneva, Switzerland , pp. 60 .
ISO/IEC ( 2009 ) ISO/IEC Directives, Part 1 Procedures for the Technical Work , 7th edn. International Organization for Standardization , Geneva, Switzerland , pp. 80 .
Jenkinson DS Powlson DS ( 1976 ) Effects of biocidal treatments on metabolism in soil. 5. Method for measuring soil biomass . Soil Biol Biochem 8 : 209 – 213 .
Kaur A Chaudhary A Kaur A Choudhary R Kaushik R ( 2005 ) Phospholipid fatty acid – a bioindicator of environment monitoring and assessment in soil ecosystem . Curr Sci 89 : 1103 – 1112 .
Kearney PC ( 1982 ) IUPAC pesticide commission report . J Assoc Off Anal Chem 65 : 1030 – 1032 .
Krsek M Wellington EMH ( 1999 ) Comparison of different methods for the isolation and purification of total community DNA from soil . J Microbiol Methods 39 : 1 – 16 .
deLiphtay JR Enzinger C Johnsen K Aamand J Soerensen SJ ( 2004 ) Impact of DNA extraction method on bacterial community composition measured by denaturing gradient gel electrophoresis . Soil Biol Biochem 35 : 1607 – 1614 .
Martin-Laurent F Philippot L Hallet S Chaussod R Germon JC Soulas G Catroux G ( 2001 ) DNA extraction from soils: old bias for new microbial diversity analysis methods . Appl Environ Microbiol 67 : 2354 – 2359 .
Marx MC Wood M Jarvis SC ( 2001 ) A microplate fluorimetric assay for the study of enzyme diversity in soils . Soil Biol Biochem 33 : 1633 – 1640 .
Marx MC Kandeler E Wood M Wermbter N Jarvis SC ( 2005 ) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions . Soil Biol Biochem 37 : 35 – 48 .
vonMersi W Schinner F ( 1991 ) An improved and accurate method for determining the dehydrogenase-activity of soils with iodonitrotetrazolium chloride . Biol Fertil Soil 11 : 216 – 220 .
Millennium Ecosystem Assessment ( 2005 ) Ecosystems and Human Well-Being: Biodiversity Synthesis . World Resources Institute , Washington, DC .
Mueller T Joergensen RG Meyer B ( 1992 ) Estimation of soil microbial biomass-C in the presence of living roots by fumigation extraction . Soil Biol Biochem 24 : 179 – 181 .
Nelson D Sommers L ( 1996 ) Total carbon, organic carbon and organic matter . Methods of Soil Analysis Part 3: Chemical Methods ( Sparks D , ed), pp. 961 – 1010 . Soil Science Society America , Madison, WA .
Niemi RM Vepsalainen M ( 2005 ) Stability of the fluorogenic enzyme substrates and pH optima of enzyme activities in different Finnish soils . J Microbiol Methods 60 : 195 – 205 .
Niemi RM Heiskanen I Wallenius K Lindstrom K ( 2001 ) Extraction and purification of DNA in rhizosphere soil samples for PCR-DGGE analysis of bacterial consortia . J Microbiol Methods 45 : 155 – 165 .
Nordgren A ( 1988 ) Apparatus for the continuous, long-term monitoring of soil respiration rate in large numbers of samples . Soil Biol Biochem 20 : 955 – 957 .
Nordgren A Baath E Soderstrom B ( 1988 ) Evaluation of soil respiration characteristics to assess heavy-metal effects on soil-microorganisms using glutamic-acid as a substrate . Soil Biol Biochem 20 : 949 – 954 .
Nortcliff S ( 2002 ) Standardisation of soil quality attributes . Agric Ecosyst Environ 88 : 161 – 168 .
Ocio JA Brookes PC ( 1990 ) An evaluation of methods for measuring the microbial biomass in soils following recent additions of wheat straw and the characterization of the biomass that develops . Soil Biol Biochem 22 : 685 – 694 .
Ohlinger R ( 1995 ) Determination of dehydrogenase activity using TTC . Methods in Soil Biology ( Schinner F Ohlinger R Kandeler E Margesin R , eds), pp. 426 . Springer-Verlag , Berlin, Germany .
Olsson PA ( 1999 ) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil . FEMS Microbiol Ecol 29 : 303 – 310 .
Pan Y Bodrossy L Frenzel P et al. ( 2010 ) Impact of inter- and intralaboratory variation on the reproducibility of microbial community analyses . Appl Environ Microbiol 76 : 7451 – 7458 .
Petric I Philippot L Abbate C et al. ( 2011 ) Inter-laboratory evaluation of the ISO standard 11063 “Soil quality – Method to directly extract DNA from soil samples” . J Microbiol Methods 84 : 454 – 460 .
Ritz K ( 2008 ) Soil as a paradigm of a complex system . Complexity and Security ( Ramsden JJ Kervalishvili PJ , eds), pp. 103 – 119 . NATO Science for Peace and Security Series: Human and Societal Dynamics (Volume 37). IOS Press , Amsterdam . ISBN: 978-1-58603-849-6.
Selmer-Olsen AR ( 1971 ) Determination of ammonium in soil extracts by an automated indophenol method . Analyst 96 : 565 – 568 .
Shelton DR Tiedje JM ( 1984 ) General-method for determining anaerobic biodegradation potential . Appl Environ Microbiol 47 : 850 – 857 .
Smalla K Cresswell N Mendoncahagler LC Wolters A VanElsas JD ( 1993 ) Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification . J Appl Bacteriol 74 : 78 – 85 .
Smith M Tiedje JM ( 1979 ) Phases of denitrification following oxygen depletion in soil . Soil Biol Biochem 11 : 261 – 267 .
Sparling GP Feltham CW Reynolds J West AW Singleton P ( 1990 ) Estimation of soil microbial C by a fumigation extraction method - use on soils of high organic-matter content, and a reassessment of the K ec -factor . Soil Biol Biochem 22 : 301 – 307 .
Spothelfer-Magaña J Thalmann A Schweikle V ( 1993 ) Methode zur bestimmung der dehydrogenaseaktivität von böden unter einsatz von Iodonitrotetrazolium-chlorid (INT): Chemische reduktion in autoklavierten sowie bestrahlten böden und der einfluß der inkubationstemperatur und -zeit . Agribiol Res 46 : 250 – 268 .
Stanford G Smith SJ ( 1972 ) Nitrogen mineralization potentials of soils . Soil Sci Soc Am J 36 : 465 – 472 .
Stemmer M Gerzabek MH Kandeler E ( 1998 ) Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication . Soil Biol Biochem 30 : 9 – 17 .
Stenberg B Johansson M Pell M Sjödahl-Svensson K Stenström J Torstensson L ( 1998 ) Microbial biomass and activities in soil as affected by frozen and cold storage . Soil Biol Biochem 30 : 393 – 402 .
Stenstrom J Stenberg B Johansson M ( 1998 ) Kinetics of substrate-induced respiration (SIR): theory . Ambio 27 : 35 – 39 .
Tabatabai MA ( 1994 ) Soil enzymes. Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties ( Weaver RW Angle S Bottomley P Bezdicek D Smith S Tabatabai MA Wollum A , eds), pp. 775 – 833 . Soil Science Society of America , Madison .
Taylor CF Paton NW Lilley KS et al. ( 2007 ) The minimum information about a proteomics experiment (MIAPE) . Nat Biotechnol 25 : 887 – 893 .
Thalmann A ( 1968 ) Zur methodik der bestimmung der dehydrogenaseaktivität im boden mittels Triphenyltetrazoliumchlorid (TTC) . Landwirtsch Forsch 21 : 249 – 258 .
Torsvik V Øvreås L Thingstad TF ( 2002 ) Prokaryotic diversity – magnitude, dynamics, and controlling factors . Science 296 : 1064 – 1066 .
Tsai YL Olson BH ( 1991 ) Rapid method for direct extraction of DNA from soil and sediments . Appl Environ Microbiol 57 : 1070 – 1074 .
van der Heijden MGA Bardgett RD van Straalen NM ( 2008 ) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems . Ecol Lett 11 : 296 – 310 .
van Elsas JD Smalla K Tebbe CC ( 2000 ) Extraction and analysis of microbial community nucleic acids from environmental matrices . Tracking Genetically Engineered Microorganisms ( Jansson JK van Elsas JD Bailey MJ , eds), pp. 29 – 61 . Landes Bioscience , Georgetown, TX .
VanBeelen P Fleuren-Kemila AK Huys MPA VanMontfort ACP VanVlaardingen PLA ( 1991 ) The toxic effects of pollutants on the mineralization of acetate in subsoil microcosms . Environ Toxicol Chem 10 : 775 – 789 .
Vance ED Brookes PC Jenkinson DS ( 1987 ) An extraction method for measuring soil microbial biomass-C . Soil Biol Biochem 19 : 703 – 707 .
Vepsäläinen M Kukkonen S Vestberg M Sirvio H Niemi RM ( 2001 ) Application of soil enzyme activity test kit in a field experiment . Soil Biol Biochem 33 : 1665 – 1672 .
Vepsäläinen M Erkomaa K Kukkonen S Vestberg M Wallenius K Niemi RM ( 2004 ) The impact of crop plant cultivation and peat amendment on soil microbial activity and structure . Plant Soil 264 : 273 – 286 .
Vogel TM Simonet P Jansson JK et al. ( 2009 ) TerraGenome: a consortium for the sequencing of a soil metagenome . Nat Rev Microbiol 7 : 252 .
Wallenstein M Weintraub M ( 2008 ) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes . Soil Biol Biochem 40 : 2098 – 2106 .
Ward TE ( 1986 ) Aerobic and anaerobic biodegradation of nitrilotriacetate in subsurface soils . Ecotoxicol Environ Saf 11 : 112 – 125 .
Watts CW Eich S Dexter AR ( 2000 ) Effects of mechanical energy inputs on soil respiration at the aggregate and field scales . Soil Till Res 53 : 231 – 243 .
Wessen E Hallin S ( 2011 ) Abundance of archaeal and bacterial ammonia oxidizers – possible bioindicator for soil monitoring . Ecol Indic 11 : 1696 – 1698 .
White DC Davis WM Nickels JS King JD Bobbie RJ ( 1979 ) Determination of the sedimentary microbial biomass by extractable lipid phosphate . Oecologia 40 : 51 – 62 .
Whitman WB Coleman DC Wiebe WJ ( 1998 ) Prokaryotes: the unseen majority . P Natl Acad Sci USA 95 : 6578 – 6583 .
Wilke BM ( 1982 ) Lead sorption and effect of lead pollution on biological-activity of different types of humus forms . Z Pflanzen Bodenk 145 : 52 – 65 .
Wilke BM Winkel B Fleischmann S Gong P ( 1998 ) Higher Plant Growth and Microbial Toxicity Tests for the Evaluation of the Ecotoxic Potential of Soils . Thomas Telford Ltd , London , pp. 345 – 354 .
Winkel B Saeger T Wilke B-M ( 1999 ) Bewertung kontaminierter Böden mit Hilfe von potentieller Nitrifikation . Ökotoxicologie-Ökosystemare Ansätze und methoden ( Oehlmann J Markert B , eds), pp. 67 – 72 . ECOMED Verlag .
Wu J Joergensen RG Pommerening B Chaussod R Brookes PC ( 1990 ) Measurement of soil microbial biomass C by fumigation extraction – an automated procedure . Soil Biol Biochem 22 : 1167 – 1169 .
Yilmaz P Kottmann R Field D et al. ( 2010 ) Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications . Nat Biotechnol 29 : 415 – 420 .
Yimaz P Gilbert JA Kniht R Amaral-Zettler L Karsh-Mizachi I Cochrane G Nakamura Y Sansone SA Glockner FO Field D ( 2011 ) The genomic standards consortium: bringing standards to life for microbial ecology . ISME J 5 : 1565 – 1567 .
Zelles L ( 1999 ) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review . Biol Fertil Soil 29 : 111 – 129 .
Zelles L Bai QY ( 1993 ) Fractionation of fatty-acids derived from soil lipids by solid-phase extraction and their quantitative-analysis by GC-MS . Soil Biol Biochem 25 : 495 – 507 .
Zhou JZ Bruns MA Tiedje JM ( 1996 ) DNA recovery from soils of diverse composition . Appl Environ Microbiol 62 : 316 – 322 .
Author notes
| Month: | Total Views: |
|---|---|
| January 2017 | 4 |
| February 2017 | 7 |
| March 2017 | 15 |
| April 2017 | 4 |
| May 2017 | 6 |
| June 2017 | 4 |
| July 2017 | 10 |
| August 2017 | 17 |
| September 2017 | 19 |
| October 2017 | 21 |
| November 2017 | 16 |
| December 2017 | 59 |
| January 2018 | 54 |
| February 2018 | 88 |
| March 2018 | 109 |
| April 2018 | 123 |
| May 2018 | 83 |
| June 2018 | 70 |
| July 2018 | 57 |
| August 2018 | 110 |
| September 2018 | 115 |
| October 2018 | 117 |
| November 2018 | 157 |
| December 2018 | 66 |
| January 2019 | 58 |
| February 2019 | 78 |
| March 2019 | 106 |
| April 2019 | 181 |
| May 2019 | 190 |
| June 2019 | 143 |
| July 2019 | 173 |
| August 2019 | 132 |
| September 2019 | 96 |
| October 2019 | 117 |
| November 2019 | 111 |
| December 2019 | 104 |
| January 2020 | 112 |
| February 2020 | 132 |
| March 2020 | 79 |
| April 2020 | 84 |
| May 2020 | 68 |
| June 2020 | 85 |
| July 2020 | 60 |
| August 2020 | 103 |
| September 2020 | 115 |
| October 2020 | 92 |
| November 2020 | 121 |
| December 2020 | 145 |
| January 2021 | 125 |
| February 2021 | 190 |
| March 2021 | 220 |
| April 2021 | 164 |
| May 2021 | 174 |
| June 2021 | 208 |
| July 2021 | 154 |
| August 2021 | 184 |
| September 2021 | 205 |
| October 2021 | 298 |
| November 2021 | 232 |
| December 2021 | 278 |
| January 2022 | 284 |
| February 2022 | 298 |
| March 2022 | 330 |
| April 2022 | 263 |
| May 2022 | 228 |
| June 2022 | 174 |
| July 2022 | 122 |
| August 2022 | 155 |
| September 2022 | 243 |
| October 2022 | 198 |
| November 2022 | 174 |
| December 2022 | 170 |
| January 2023 | 136 |
| February 2023 | 109 |
| March 2023 | 118 |
| April 2023 | 118 |
| May 2023 | 118 |
| June 2023 | 77 |
| July 2023 | 153 |
| August 2023 | 124 |
| September 2023 | 165 |
| October 2023 | 164 |
| November 2023 | 161 |
| December 2023 | 172 |
| January 2024 | 215 |
| February 2024 | 154 |
| March 2024 | 209 |
| April 2024 | 183 |
| May 2024 | 164 |
| June 2024 | 133 |
| July 2024 | 150 |
| August 2024 | 48 |
Email alerts
Citing articles via.
- Recommend to your Library
- Journals Career Network
Affiliations
- Online ISSN 1574-6941
- Print ISSN 0168-6496
- Copyright © 2024 Federation of European Microbiological Societies
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment
1 National Engineering Laboratory for Improving Quality of Arable Land, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing, China
Minggang Xu
Mohammad eyakub ali, wenju zhang, yinghua duan.
2 Qiyang Agro-ecosystem of National Field Experimental Station, Hunan, China
Associated Data
All relevant data are within the paper and its Supporting Information files.
The effects of soil type and organic material quality on the microbial biomass and functional diversity of cropland soils were studied in a transplant experiment in the same climate during a 1-year field experiment. Six organic materials (WS: wheat straw, CS: corn straw, WR: wheat root, CR: corn root, PM: pig manure, CM: cattle manure), and three contrasting soils (Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem) were chosen. At two time points (at the end of the 1st and 12th months), soil microbial biomass carbon (C) and nitrogen (N) (MBC and MBN) and Biolog Ecoplate substrate use patterns were determined, and the average well color development and the microbial functional diversity indices (Shannon, Simpson and McIntosh indices) were calculated. Organic material quality explained 29.5–50.9% of the variance in MBC and MBN when compared with the minor role of soil type (1.4–9.3%) at the end of the 1st and 12th months, and C/N ratio and total N of organic material were the main parameters. Soil properties, e.g., organic C and clay content were the predominant influence on microbial functional diversity in particular at the end of the 12th month (61.8–82.8% of the variance explained). The treatments of WS and CS significantly improved the MBC and microbial functional diversity indices over the control in the three soils in both sampling periods ( P < 0.05). These results suggest that the application of crop straw is a long-term effective measure to increase microbial biomass, and can further induce the changes of soil properties to regulate soil microbial community.
Introduction
Soil microorganisms drive the turnover of exogenous organic materials into soil organic matter [ 1 ]. The quality of applied organic materials can regulate microbial abundance and function [ 2 – 4 ]. In China, large amounts of agricultural byproducts are produced because high agricultural productivity is being pursued to meet the food demands of the huge population. In China in 2011, 863 million tons of crop straw and 3 trillion tons of livestock manure were produced [ 5 – 6 ]. These excessive byproducts have created a series of negative environmental effects, such as atmospheric pollution, water eutrophication and so on. In agricultural systems, the return of organic materials to the soil is the most prevalent practice to maintain or improve soil fertility. However, the size and function of microorganisms is different in different soils, for example, high content of soil organic matter is generally associated with high microbial abundance and diversity [ 7 – 8 ]. Increased understanding of the size and function of microorganisms after application of different organic materials in different types of agricultural soil is therefore helpful to clarify the effect of organic materials and soil types on microbial characteristics.
Soil microbial biomass carbon (C) and nitrogen (N) (MBC and MBN) reflect microbial size and soil fertility status, and they act as the living nutrient pool in soil [ 9 ]. Soil microbial functional diversity is linked with the stability of soil microbial communities and levels of soil biodiversity [ 10 ]. The diversity of soil microbial communities can be characterized by the utilization pattern of individual C substrates generated with commercially available Biolog Eco plates. These community-level physiological profiles (CLPPs) have provided a rapid means for evaluating the structure and species composition of soil microbial communities. The average well color development (AWCD) and the functional diversity indices, including Shannon ( H’ ), Simpson ( D ) and McIntosh ( U ) indices, are important diagnostic indicators of soil quality [ 11 ]. Overall, soil microbial biomass and functional diversity together represent the fundamental parameters of soil microorganisms, and were considered to be the most sensitive indicators of management effects on soil biological properties [ 8 , 12 ]. The analysis of soil microbial characteristics can indicate the status of soil fertility and ecosystem function.
The quality of organic materials affects the microbial biomass and community structure [ 13 – 16 ]. Microbial biomass carbon and Shannon’s diversity index after amendment with labile organic materials with low lignin content were significantly higher than that after amendment with recalcitrant organic materials with high lignin content [ 16 – 17 ]. In agricultural systems, the available organic materials generally include crop residues and livestock manures; crop residues are characterized by higher C:N ratio and lower available nutrient content in comparison with manure [ 15 ]. Generally, the microbial biomass or functional diversity after amendment with crop straws was lower than that with manure in agricultural soils because of the low availability of C sources and nutrients in crop residues [ 13 , 15 – 18 ]. To date, most studies of soil microbial characteristics with different organic materials amendment concentrated mainly on a certain soil or different soils under controlled laboratory conditions [ 17 , 19 ]; little information is reported about comparative studies of microbial characteristics dynamics in soils developed from different parent materials after amendment with different organic materials under field conditions.
Soil properties, such as parent material, soil organic matter, pH and clay content can also influence soil microbial biomass and functional diversity [ 19 – 21 ]. Soil parent material provides the basic nutritional environment for development of the microbial community [ 22 – 23 ], and during soil formation the soil microbial communities can be changed [ 19 , 21 ]. Soil organic matter provides energy to microbes, and soil with higher content of SOM generally has higher microbial biomass and functional diversity [ 7 , 17 , 24 – 26 ]. Soil pH plays an important role in shaping microbial community composition [ 27 – 30 ]; soil pH was negatively correlated with soil biomass and positively correlated with AWCD [ 11 , 13 ]. Soil texture can also affect the soil nutrient status and water content, thus affecting the living environment and metabolic activity of microorganisms [ 31 – 32 ]. Ranjard and Richaume (2001) [ 33 ] found that 40–70% of the bacteria were located in the 2–20 and < 2 μm aggregates. Consequently, the comparison of microbial characteristics in different soil types can improve our understanding of the influence of soil properties on microbes.
In China, the Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem are the typical intensive cropland soils. Currently, these Chinese cropland soils have the obvious trend of acidification because of excessive N fertilizer application when compared with those soils 30 years ago [ 34 ]. Ferralic Cambisol is found in the subtropical region with an acidic soil environment [ 35 ], Calcaric Cambisol is found in the warm temperate region with a weak basic or neutral soil environment, and Calcaric Cambiso is found in the cold temperate region with a weak acidic or neutral soil environment. To better compare the effects of exogenous organic materials and soil type on the microbial characteristics and to eliminate the effect of climate factors, Calcaric Cambisol and Luvic Phaeozem were moved to the subtropical region to accentuate the effects of global warming and soil acidification. The objectives of the present study were therefore (1) to explore the changes in microbial biomass and functional diversity during the decomposition of organic materials in different soil types, and (2) to quantify the contributions of soil type and quality of organic materials to microbial biomass and functional diversity.
Materials and methods
Soils collection.
Three typical cropland soils including Ferralic Cambisol, Calcaric Cambisol and Luvic Phaeozem (FAO classification) were collected from the national long-term monitoring stations of soil fertility which were established by Qiyang Agro-ecosystem of National Field Experimental Station, Henan Academy of Agricultural Sciences and Jilin Academy of Agricultural Sciences, respectively. The Ferralic Cambisol developed from the quaternary red soil was located in Qiyang County, Hunan Province; this region has a subtropical climate, with an annual average temperature of 18°C and an average annual rainfall of 1255 mm. The Calcaric Cambisol developed from alluvial sediments of the Yellow River was located in Yuanyang County, Henan Province; this region has a temperate sub-humid climate, with an annual average temperature of 14.5°C and an average annual rainfall of 450–600 mm. The Luvic Phaeozem derived from the quaternary loess sediments was located in Gongzhuling County, Jilin Province; this region is characterized by temperate sub-humid climate, with an annual average temperature of 4–5°C and an average annual rainfall of 450–600 mm. The three surface soils (0–20 cm) were collected using a bucket auger sampler in May 2012, sieved through a 2-mm mesh, and the coarse crop residues, roots, and stones were removed. The soil physicochemical properties were shown in Table 1 and the average monthly rainfall and temperature of the study site during the sampling period were shown in Fig 1 .
| Properties | Ferralic Cambisol | Calcaric Cambisol | Luvic Phaeozem |
|---|---|---|---|
| Location | 111°52′N, 26°45′E | 113°41′N, 35°00′E | 124°48′N, 43°30′E |
| Organic carbon (g kg ) | 10.1±0.1b | 6.3±0.1c | 15.6±0.2a |
| Total N (g kg ) | 1.2±0.1a | 0.7±0.1b | 1.4±0.2a |
| Total P (g kg ) | 0.9±0.1a | 0.7±0.1b | 0.6±0.1c |
| Total K (g kg ) | 12.8±0.1b | 21.3±0.3a | 22.9±0.3a |
| pH (soil:water = 1:2.5) | 5.2±0.1b | 8.3±0.2a | 5.9±0.1b |
| Sand (%) | 18.8±0.3c | 67.1±1.1a | 38.7±0.6b |
| Silt (%) | 31.9±0.4a | 18.7±0.2c | 28.7±0.4b |
| Clay (%) | 43.9±0.8a | 10.1±0.2c | 29.3±0.5b |
Values are mean ± standard error of three replicates. Different letters in the same row indicate significant difference at P <0.05 among three soils.

Preparation of organic material
Six kinds of organic materials were chosen, including wheat ( Triticum aestivum L.) straw (WS), corn ( Zea mays L.) straw (CS), wheat root (WR), corn root (CR), pig manure (PM), and cattle manure (CM). All the organic materials were oven-dried at 60°C, and passed through a 2-mm sieve. The chemical characteristics of these organic materials were shown in Table 2 .
| Organic materials | Total C (g kg ) | Total N (g kg ) | C: N ratio | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| Wheat straw (WS) | 450.7±16a | 5.1±0.8c | 88.0±5.1a | 36.4±0.0b | 30.6±0.2b | 7.8±0.1c |
| Corn straw (CS) | 461.8±14a | 5.2±0.6c | 89.0±4.2a | 36.5±0.2b | 33.2±0.0a | 4.0±0.0e |
| Wheat root (WR) | 329.7±17d | 3.7±0.3d | 89.6±4.3a | 28.2±1.6c | 25.0±1.8c | 6.4±0.3d |
| Corn root (CR) | 473.3±15a | 5.8±0.5c | 81.5±3.8a | 40.2±0.2a | 33.2±0.2a | 6.4±0.3d |
| Pig manure (PM) | 358.0±11c | 11.6±0.8b | 31.0±3.1b | 28.0±0.2c | 23.8±0.1c | 12.1±0.0a |
| Cattle manure (CM) | 396.9±12b | 18.0±0.9a | 22.0±2.8b | 19.7±0.1d | 25.6±0.4c | 8.6±0.2b |
Values are mean ± standard error of three replicates. Different letters in the same column indicate significant difference at P <0.05 among different materials.
Experimental design
The experiment was carried out from June 5, 2012 to June 5, 2013 in the long-term experimental station of the Chinese Academy of Agricultural Sciences, Qiyang County (111°52′32″N, 26°45′12″E), Hunan Province. Before the experiment, both Calcaric Cambisol from Zhengzhou and Luvic Phaeozem from Gongzhuling were moved to Qiyang County.
Soil type was the main plot factor and organic material was the subplot factor in a split-plot design with six replicates; three replicates were sampled at the end of the 1st month, and the other three replicates were sampled at the end of the 12th month. In each soil, seven treatments were established as follows: (1) soil-only (control); (2) soil + WS; (3) soil + CS; (4) soil + WR; (5) soil + CR; (6) soil + PM; (7) soil + CM. A total of 126 nylon bags (20 × 15 cm 2 , 0.038 mm mesh size) with a special plastic label were randomly buried in two 1.5 × 1.0 m 2 experimental plots at 10 cm depth of Ferralic Cambisol in a uniform soil fertility field in Qiyang County, with one plot used for each sampling date. In each bag, 200 g (oven-dried basis) experimental soil (Ferralic Cambisol, Calcaric Cambisol, Luvic Phaeozem) was thoroughly mixed with organic material at a ratio of 15 g C kg −1 soil, which was equivalent to 34 t C ha −1 returned to the soil. The amount of different organic materials in each bag was described in S1 Table . During the experimental period, no crops were planted in the plots, and weeds were removed regularly by hand to decrease the effect of weed roots on the nylon bags. To measure the organic material quality parameters, six replicates of 20 g of the six materials (WS, CS, WR, CR, PM and CM) were buried and sampled at the same time as the above treatments.
Sampling and analysis
On the sampling day, each bag was weighed after the attached soil on the outer wall of the bag was carefully removed. Part of the fresh soil in the bag was taken to determine microbial CLPPs, MBC and MBN, while the remaining soil was air-dried to determine soil organic C (SOC), total N, and pH. Total fiber content of organic material (cellulose, hemicellulose and lignin), total organic C, and total organic N were determined.
BIOLOG analysis
Microbial CLPPs in soil were determined by Biolog Eco plates (Biolog, Hayward, CA, USA). Briefly, 5 g of fresh soil was shaken in 45 ml of sterile saline solution (0.85% NaCl w/v) for 30 min at the rate of 180 rpm, and then the mixture was diluted 100-fold. Aliquots of 150 μl of the 10 −3 suspension was incubated in each well of Biolog Eco plates at 28°C and the absorbance was measured at 590 nm with an Emax precision microplate reader (Biolog, Hayward, CA, USA). The readings at 96 h incubation collected by Microlog Rel. 4.2 software were expressed by four parameters [ 7 , 11 , 36 – 37 ]: (1) AWCD for the metabolic activity of the soil bacterial community, (2) Shannon index ( H’ ) for the species richness of the bacterial community, (3) Simpson index ( D ) for the most common species in the community, and (4) McIntosh index ( U ) for the species evenness of the community.
The AWCD is calculated to reflect the utilization of single C sources by soil microorganisms:
where Ci is the absorbance of well i and R is the absorbance of the control well. When Ci—R <0 or Ci—R <0.06, the values were set to 0 [ 38 ].
The functional diversity indices were calculated as described by Zhong et al. [ 11 ]:
where Pi is the ratio of activities on each substrate to the sum of activities on all substrates and ni is the activities on each substrate.
Microbial biomass
Microbial biomass C and N were determined by the fumigation-extraction method [ 39 ]. A 20-g subsample of soil (oven-dried basis) was fumigated by exposing the soil to alcohol-free CHCl 3 vapor in a sealed vacuum desiccator for 24 h. The fumigated soil was evacuated repeatedly in a clean empty desiccator until the odor of CHCl 3 was not detected, and then extracted with 80 ml 0.5 M K 2 SO 4 (soil:K 2 SO 4 = 1:4) for 30 min. The extraction of non-fumigated soil was the same as that of the fumigated soil. Microbial biomass C and N were estimated by the difference between the total organic C or total N in the fumigated and non-fumigated extracts with a conversion factor (K EC ) of 0.38 and (K EN ) of 0.45 [ 40 – 41 ], respectively.
Physicochemical analysis of soil and organic material
Soils and organic materials were analyzed for organic C and total C by dichromate oxidation and total N by Kjeldahl digestion. Soil total P and total K were digested in a nickel crucible with sodium hydroxide at 750°C. Soil available P was extracted with 0.5 M NaHCO 3 . Soil total P and available P were determined by the molybdenum-blue method at a wavelength of 880 nm. Soil available K was extracted with 1 M NH 4 OAc. Soil total K and available K were determined using atomic absorption spectrophotometry. Soil pH was determined in water (soil: water = 1: 2.5). Soil clay, silt and sand were determined by the pipette method. Total fiber content of organic material (cellulose, hemicellulose and lignin) was determined by the method described by van Soest [ 42 ].
Statistical analysis
Statistical analysis of all variables was carried out using the SPSS 16.0 software package. To evaluate the primary factors influencing microbial parameters, we analyzed MBC, MBN, AWCD, H’ , D and U using a two-way analysis of variance (ANOVA) with soil and organic material types as independent factors and permitted to interact. A one-way ANOVA was used to determine the differences of soil properties, chemical characteristics of organic material, and the above microbial parameters among organic material treatments at each soil. The differences among treatments with separation of means by Tukey’s HSD (α = 0.05) test at P < 0.05. Principal component analysis (PCA) of the Ecoplate data was performed to characterize the effect of different organic materials on soil microbial community functions, and the differences of the factor scores of the first principal component (PC1) axis among organic material treatments at each soil were tested using a one-way ANOVA by Tukey’s HSD (α = 0.05) test at P < 0.05. Stepwise multiple regression analysis was applied to determine the key factors influencing microbial properties.
Microbial biomass carbon and nitrogen (MBC and MBN)
At the end of the 1st month, the contributions of soil type and organic material type were significant in explaining the variance in MBC and MBN, and explained 6.9 and 43.6% of the variance in MBC, as well as 9.3 and 50.9% of the variance in MBN, respectively ( P < 0.05; Table 3 ). Significantly higher MBC and MBN were found in Calcaric Cambisol and Luvic Phaeozem than that in Ferralic Cambisol regardless of organic material type ( P < 0.05, Fig 2A and 2C ). When compared with the control, all organic material treatments significantly increased the MBC while only the CM and PM treatments significantly increased the MBN in the three soils ( P < 0.05, Fig 2A and 2C ).
| Parameters | Source of variation | df | %SS | F | %SS | F | ||
|---|---|---|---|---|---|---|---|---|
| 1 month | 12 month | |||||||
| Soil | 2 | 6.9 | 9.9 | <0.05 | 1.4 | 0.6 | 0.561 | |
| Organic material | 5 | 43.6 | 25.2 | <0.05 | 45.3 | 7.5 | <0.05 | |
| Soil × Organic material | 10 | 37.1 | 12.2 | <0.05 | 9.7 | 0.8 | 0.630 | |
| Residuals | 36 | 12.5 | 43.6 | |||||
| Soil | 2 | 9.3 | 8.1 | <0.05 | 3.0 | 1.5 | 0.245 | |
| Organic material | 5 | 50.9 | 17.9 | <0.05 | 29.5 | 5.7 | <0.05 | |
| Soil × Organic material | 10 | 19.3 | 3.4 | <0.05 | 30.2 | 2.9 | <0.05 | |
| Residuals | 36 | 20.5 | 37.4 | |||||
| Soil | 2 | 24.8 | 181.3 | <0.05 | 80.0 | 1226.0 | <0.05 | |
| Organic material | 5 | 38.4 | 112.2 | <0.05 | 5.4 | 111.9 | <0.05 | |
| Soil × Organic material | 10 | 34.3 | 50.0 | <0.05 | 13.3 | 60.1 | <0.05 | |
| Residuals | 36 | 2.5 | 1.2 | |||||
| ) | ||||||||
| Soil | 2 | 74.4 | 380.6 | <0.05 | 65.7 | 178.5 | <0.05 | |
| Organic material | 5 | 13.7 | 28.1 | <0.05 | 11.1 | 12.1 | <0.05 | |
| Soil × Organic material | 10 | 8.4 | 8.6 | <0.05 | 16.6 | 9.0 | <0.05 | |
| Residuals | 36 | 3.5 | 6.6 | |||||
| ) | ||||||||
| Soil | 2 | 45.3 | 74.1 | <0.05 | 61.8 | 122.8 | <0.05 | |
| Organic material | 5 | 22.7 | 14.7 | <0.05 | 10.1 | 8.0 | <0.05 | |
| Soil × Organic material | 10 | 21.3 | 6.9 | <0.05 | 19.1 | 7.6 | <0.05 | |
| Residuals | 36 | 10.7 | 9.0 | |||||
| Soil | 2 | 34.2 | 250.1 | <0.05 | 82.8 | 1348.5 | <0.05 | |
| Organic material | 5 | 40.3 | 118.1 | <0.05 | 5.5 | 35.8 | <0.05 | |
| Soil × Organic material | 10 | 23.1 | 33.8 | <0.05 | 10.6 | 34.6 | <0.05 | |
| Residuals | 36 | 2.5 | 1.1 | |||||

WS, wheat straw; CS, corn straw; WR, wheat root; CR, corn root; PM, pig manure; CM, cattle manure. Different letters indicate significant differences at P < 0.05 among different materials in the same soil.
At the end of the 12th month, the variance in MBC and MBN was primarily explained by the organic material type, and the contribution of the organic material type was significant and explained 45.3% of the variance in MBC and 29.5% of the variance in MBN ( P < 0.05, Table 3 ). The WS, CS, WR and CR treatments significantly increased the MBC while only the WS and CS treatments significantly increased the MBN when compared with the control in the three soils ( P < 0.05, Fig 2B and 2D ). When compared with the end of the 1st month, the MBC at the end of the 12th month decreased by 21.5–28.7%, and the MBN at the end of the 12th month increased by 62.9–143.7% in the three soils ( Fig 2 ).
Metabolic activity and microbial functional diversity
At the end of the 1st month, the contributions of soil type and organic material type were significant in explaining the variance in microbial functional diversity ( P < 0.05, Table 3 ). The AWCD and McIntosh index was primarily explained by the organic material species (38.4 and 40.3%, respectively), and the Shannon and Simpson indices were primarily explained by soil type (74.4 and 45.3%, respectively). The microbial functional diversity of Ferralic Cambisol and Luvic Phaeozem in all organic material treatments was significantly increased when compared with the control ( P < 0.05), while only the WS and CS treatments significantly increased all functional diversity indices in Calcaric Cambisol when compared with the control ( P < 0.05, Fig 3A, 3C, 3E and 3G ).

At the end of the 12th month, the contributions of soil type and organic material type were also significant in explaining the variance in the microbial functional diversity ( P < 0.05, Table 3 ), with 61.8–82.8% of the variances in functional diversity primarily explained by soil type ( P < 0.05, Table 3 ). The WS and WR treatments significantly increased the AWCD, Shannon and McIntosh indices in Ferralic Cambisol and Luvic Phaeozem when compared with the control, and all organic material treatments increased the functional diversity indices in Calcaric Cambisol when compared with the control ( P < 0.05, Fig 3B, 3D, 3F and 3H ).
Carbon substrate utilization patterns of soil microbial communities
To reduce the dimensionality of the data set, a PCA was performed to compare the effect of different organic material treatments on the Biolog Ecoplate utilization patterns of C substrates in the three soils. At the end of the 1st month, the ANOVA for principal component 1 (PC1) indicated that the patterns of substrate utilization between the organic materials and the control treatments were significantly different in Ferralic Cambisol and Luvic Phaeozem ( P < 0.05), and that they were significantly different between the WS and CS treatments and the control in Calcaric Cambisol ( P < 0.05, Fig 4A–4C ). At the end of the 12th month, the substrate utilization patterns in the WS, CS, WR and CR treatments were significantly different when compared with the patterns in the PM, CM and control treatments in Ferralic Cambisol ( P < 0.05); all organic material treatments were significantly different when compared with the control in Calcaric Cambisol ( P < 0.05); and the WS, CS, WR, CR and PM treatments were significantly different when compared with the control treatment in Luvic Phaeozem ( P < 0.05, Fig 4D–4F ).

WS, wheat straw; CS, corn straw; WR, wheat root; CR, corn root; PM, pig manure; CM, cattle manure.
A high Pearson correlation coefficient (> 0.6) for PC1 in the organic material treatments was shown in Table 4 . At the end of the 1st month, the C substrate use pattern was primarily associated with increased utilization of carbohydrates, amino acids and polymer in Ferralic Cambisol; carbohydrates, amino acids, carboxylic acid, polymer and amine in Calcaric Cambisol; carbohydrates, carboxylic acids, amino acid and polymer in Luvic Phaeozem. At the end of the 12th month, the C substrate use pattern was changed in the three soils. It was associated with increased utilization of carbohydrates, amino acids and amine in Ferralic Cambisol and Calcaric Cambisol, and carbohydrates, amino acids, carboxylic acids and polymer in Luvic Phaeozem.
| Type | Substrate name | Ferralic Cambisol | Calcaric Cambisol | Luvic Phaeozem | Ferralic Cambisol | Calcaric Cambisol | Luvic Phaeozem |
|---|---|---|---|---|---|---|---|
| 1 month | 12month | ||||||
| β-methyl-D-Glucoside | 0.880 | 0.880 | 0.949 | 0.915 | |||
| ᴅ-Galactonic acid lactone | 0.736 | 0.847 | 0.764 | 0.668 | 0.945 | 0.735 | |
| ᴅ-Xylose | |||||||
| ᴅ-Galacturonicacid | 0.888 | 0.912 | 0.608 | 0.966 | 0.949 | ||
| γ-Lactone | |||||||
| i-Erythritol | 0.693 | 0.630 | |||||
| ᴅ-Mannitol | 0.824 | 0.932 | 0.833 | 0.883 | 0.931 | ||
| -acetyl-ᴅ-Glucosamine | 0.864 | 0.885 | 0.680 | 0.945 | 0.929 | ||
| ᴅ-Glucosaminic acid | 0.783 | ||||||
| ᴅ-cellobiose | 0.911 | 0.735 | |||||
| α-ᴅ-Glucose-1-phosphate | 0.877 | 0.897 | 0.953 | 0.812 | |||
| α-ᴅ-lactose | 0.782 | 0.900 | 0.809 | 0.864 | |||
| ᴅ,L-α-Glyce | 0.767 | 0.849 | 0.803 | ||||
| L-Arginine | 0.602 | ||||||
| L-Asparagine | 0.837 | 0.934 | 0.686 | 0.939 | |||
| L-Phenylalanine | 0.724 | 0.713 | 0.741 | 0.711 | |||
| L-Serine | 0.871 | 0.906 | 0.946 | 0.882 | |||
| L-threonine | 0.766 | 0.733 | |||||
| Glycyl-L-glutamic | 0.740 | 0.899 | 0.918 | 0.752 | |||
| Pyruvic acid methyl ester | |||||||
| γ-Hydroxybutyric acid | 0.750 | 0.946 | |||||
| Itaconic acid | |||||||
| α-Ketobutyric acid | |||||||
| ᴅ-Malic acid | 0.714 | 0.686 | |||||
| Tween 40 | 0.617 | ||||||
| Tween 80 | 0.703 | 0.687 | |||||
| α-Cyclodextrin | |||||||
| Glycogen | 0.681 | ||||||
| 2-Hydroxy benzoic Acid | |||||||
| 4-Hdroxy benzoic acid | |||||||
| Phenylethyl-anime | |||||||
| Putrescine | 0.853 | 0.729 | 0.771 | ||||
The relationships among microbial properties, organic material quality and soil physicochemical properties
The C/N ratio and N content of organic materials significantly affected the MBC and MBN at the end of the 1st and 12th months ( P < 0.05), and soil clay significantly affected MBC at the end of the 1st month ( P < 0.05, Table 5 ). At the end of the 1st month, soil clay content significantly influenced AWCD and U , pH significantly influenced D , and total nitrogen significantly influenced H’ ( P < 0.05). The lignin content of organic materials significantly influenced H’ and D at the end of the 1st month ( P < 0.05). At the end of the 12th month, the soil organic C (SOC) and C/N ratio of organic materials significantly influenced AWCD, H’ , D and U ( P < 0.05), and the clay content significantly influenced H’ and D ( P < 0.05).
| Regression equation | |
|---|---|
| MBC = 467.895–1.526 C/N—1.347 clay | 0.38 |
| MBN = 13.087 + 67.799 OTN | 0.44 |
| AWCD = 0.267 + 0.010 clay | 0.24 |
| Shannon index ( ) = 5.038–2.801 STN + 0.127 SOC– 0.200 lignin | 0.78 |
| Simpson index ( ) = 1.057–0.018 pH– 0.021 lignin | 0.48 |
| McIntosh index ( ) = 3.300 + 0.078 clay—5.653 OTN | 0.36 |
| MBC = 154.602 + 3.788 C/N | 0.43 |
| MBN = 21.122 + 0.870 C/N | 0.22 |
| AWCD = 1.105–0.081 SOC+ 0.010 C/N | 0.84 |
| Shannon index ( ) = 2.921–0.077 SOC + 0.014 C/N + 0.006 clay | 0.73 |
| Simpson index ( ) = 0.938–0.007 SOC + 0.001 C/N + 0.001 clay | 0.68 |
| McIntosh index ( ) = 7.769–0.524 SOC + 0.073 C/N | 0.86 |
Soil properties: SOC, soil organic carbon; STN, soil total nitrogen; clay; pH; Organic material quality: OTN, total nitrogen; C/N ratio; lignin.
Effects of soil properties and organic materials quality on microbial biomass
Soil microbial biomass represents the amount of microbes in soil, and was successfully used to detect short-term changes in soil functioning to predict organic C accumulation in soil under organic management [ 20 ]. The quality of organic material, e.g., the C availability, the C/N ratio and N content, determines the size of the microbial biomass [ 13 , 43 – 45 ] ( Table 3 ). Carbon sources can provide energy for microorganisms [ 46 – 47 ], and microorganisms can grow rapidly when they encounter abundant C sources, e.g., the significant increase in MBC in organic materials amendment treatments when compared with the control treatment in the three soils at the end of the 1st month ( Fig 2 ). The C/N ratio of organic materials has generally been shown to be a good predictor of the decomposition of organic materials [ 45 , 48 ], and organic materials with low C/N ratio can supply sufficient nutrients for microbes [ 49 – 50 ], which was shown by the significantly higher MBC and MBN in manure treatments than those in crop residue treatment at the end of the 1st month ( Table 2 , Fig 2 ). Nevertheless, at the end of the 12th month ( Fig 2 ) the crop residue amendments with high C/N ratio induced significantly higher MBC and MBN than the manure amendments. Generally, soil N immobilization occurred with organic materials amendment [ 47 , 51 ]. As the experiment proceeded, the amount of available C and N sources decreased and further entered the environment, e.g., as C and N gaseous emissions, dissolved organic C and nitrate leaching under the high precipitation in the experimental subtropical region, especially in the manure amendment treatments; large amounts of easily decomposable and passive decomposable C sources and nutrients were activated by microbial metabolism, and then these activated C sources and nutrients can be easily lost. Conversely, the N limitation was more serious in crop material treatments with high C/N ratio than in manure treatments ( S1 Fig ) [ 46 ]; the immobilized N induced by crop materials can be recycled in microorganisms with crop materials decomposition [ 47 , 52 ]. A 15 N-tracer experiment also demonstrated that organic materials with high C/N ratio prolong nutrient retention in soil through microbial metabolism [ 47 ]. The calculated ratio of MBC to MBN between the 1st and 12th months in this study supports the above phenomenon.
Soil properties had less influence on microbial biomass when compared with the organic material quality, with significant effects only observed at the end of the 1st month ( Table 3 ). In the present study, organic materials amendment might have completely obscured the effect of soil properties on microbial biomass. Generally, soils with high SOC content had high microbial biomass [ 25 – 26 , 53 – 54 ], and nutritional stress might occur when SOC was less than 1% [ 53 ]. When compared with the other two soils in our study ( Table 1 ), Luvic Phaeozem had high microbial biomass because SOC in Luvic Phaeozem is 1.5–2.5 times that of the other two soils. Further, stepwise multiple regression analysis showed that clay content was negatively correlated with MBC after the addition of organic materials at the end of the 1st month. Müller et al. [ 55 ] reported that the clay protective effect of nutrients on microbial biomass was limited, and the increase in clay content could not improve the response of microorganisms to organic material amendment when clay content was > 25%; for example, Ferralic Cambisol had higher clay content than the other two soils in the present study. Meanwhile, the low pH in the Ferralic Cambisol (pH = 5.2) would reduce the utilization of labile substrate by soil microbes [ 56 – 57 ] because of the toxic exchangeable Al in low pH soil [ 58 ]; however, the integrated effect of SOC, clay content and organic materials amendment could affect the response of microbial biomass to pH as shown by the non-significance of pH in explaining microbial biomass in the stepwise multiple regression analysis.
Effects of soil properties and organic materials quality on microbial functional diversity
The average well color development (AWCD) and the functional diversity indices including Shannon, Simpson and McIntosh indices were often used to investigate the general structure and functional potential of soil microbial communities [ 13 , 24 ]. The integrated effect of soil type and organic material amendment significantly ( P < 0.05) affected the microbial functional diversity. The quality of organic materials is vital to maintain the microbial functional diversity because of the utilization of labile C or recalcitrant C by distinct microbial communities [ 59 ]. Lignin is resistant to biodegradation and higher lignin content depresses microbial metabolism; this resulted in the negative correlation between lignin content and the diversity indices (Shannon and Simpson indices) in different organic material treatments at the end of the 1st month [ 17 , 45 ]. At the end of the 12th month in the present study, the microbial functional diversity indices were positively correlated with C/N ratio of organic materials ( Table 5 ), and the microbial communities in crop residue treatments were separated from those in the control treatment in the three soils ( Fig 4 ); this is because the decomposable C sources from crop residue, including cellulose and hemicellulose, and the lower lignin content in crop residues when compared with that in manures supported high microbial functional diversity [ 17 , 44 , 60 ].
Soil properties were more important than organic material properties in explaining the microbial functional diversity as shown in Table 3 [ 13 , 18 – 19 , 24 ]. At the end of the 1st month, the increase in AWCD and McIntosh index with increased clay content was because silt and clay particles generally supported larger and more diverse microbial communities than sand particles [ 61 ]. High soil N content negatively affected soil microbial communities and led to a decrease in the microbial functional diversity by altering the supply and quality of organic matter [ 27 , 62 ]; which resulted in significantly lower Shannon index in Ferralic Cambisol and Luvic Phaeozem than that in Calcaric Cambisol. Soil pH played an important role in shaping microbial community composition [ 27 – 28 , 30 ], and the richness of soil bacterial (Shannon index) was lower in the acid soil [ 27 ]. The present study was not all consistent with the previous reports, although the Shannon index in Ferralic Cambisol and Luvic Phaeozem with lower initial pH ( Table 1 ) was lower than that in Calcaric Cambisol at the end of the 1st month ( Fig 3C ). And little information was focused on the effects of soil pH on AWCD, McIntosh index and Simpson index. The high precipitation in the study site would leach the soluble acid ions into the litter bags, thus limiting organic matter availability and inhibiting microbial metabolism ( Fig 1 ) [ 63 – 64 ]. As a result, AWCD and McIntosh index were low in Calcaric Cambisol because of its high initial pH ( Table 1 ) at the end of the 1st month. As the experiment proceeded, the soil microbial community in Calcaric Cambisol adapted to the experimental environment, and the low nutrient content in Calcaric Cambisol may encourage the microbes to assimilate exogenous C resources from the added organic materials [ 13 , 65 ]; hence, significantly higher microbial functional diversity indices were found in Calcaric Cambisol at the end of the 12th month when compared with those in the 1st month. When compared with the other two soils, Luvic Phaeozem soil had the highest SOC content ( Table 1 ) and significantly lower functional diversity indices at both sampling dates ( Fig 3 ), and the reasons were that (1) Luvic Phaeozem per se had the lowest functional diversity as shown the control treatment ( Fig 3 ), (2) soil with high organic matter has sufficient available C sources for microbial assimilation, and showed reduced assimilation of exogenous C sources by microbes when compared with the Ferralic Cambisol and Calcaric Cambisol with lower organic matter content [ 13 , 65 ]. In addition, soil microbial communities were largely affected by historical factors such as geographic location and soil type due to microbes dwelling in soil [ 20 , 23 , 66 – 67 ]. It has been showed that soil microbial diversity decreased with the increase of latitude and was positively correlated with air temperature [ 68 ], and Luvic Phaeozem in this study was developed from the highest latitude and the annual average temperature (4–5°C) in its local region was lower than the other two soils. Hence, it explained the lower functional diversity in Luvic Phaeozem than the other two soils. Though, Luvic Phaeozem soil transfered from the temperate sub-humid region to the subtropical region, however, the short term effect of climate in this study (≤ 1 year) was not enough to alter the initial microbial communities because it had been reported that no significant responses of climate change on microbial communities within less than 10 years [ 69 ].
Conclusions
Both organic material quality and soil type affected soil microbial characteristics. Organic material quality played a predominant role in controlling the microbial biomass at both sampling periods, and the main parameters of organic matter were C/N ratio and N content. Although manures, with low C/N ratio and high nitrogen content, significantly increased microbial biomass when compared with crop residues at the end of the 1st month ( P < 0.05), the crop residues significantly increased the microbial biomass when compared with manures at the end of the 12th month ( P < 0.05). After the easily available C was exhausted, soil properties regulated the microbial functional diversity, and the main parameters of soil properties were soil organic C and clay content. When compared with the manures, crop residues, in particular straws with low lignin and high C/N ratio, significantly increased the functional diversity indices at both sampling periods ( P < 0.05). This study suggests that the application of straw is a long-term effective measure to increase microbial biomass, and can further induce the changes of soil properties to regulate soil microbial community.
Supporting information
Acknowledgments.
This work was supported by the National Natural Science Foundation of China (41571298), and the International (Regional) Joint Research Program (41620104006). We thank Catherine Dandie, PhD, from Liwen Bianji, Edanz Group China ( www.liwenbianji.cn/ac ), for editing the English text of a draft of this manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (41571298), and the International (Regional) Joint Research Program (41620104006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Author Biographies
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Modeling bibb lettuce nitrogen uptake and biomass productivity in vertical hydroponic agriculture.

1. Introduction
1.1. fertilizer shortcomings, 1.2. controlled environment agriculture and wastewater, 1.3. modeling in hydroponics, 1.4. objectives, 2. materials and methods, 2.1. experimental configuration, 2.1.1. controlled environment chambers, 2.1.2. vertical nutrient film technique (nft) hydroponic systems, 2.1.3. seedling germination and transplantation, 2.1.4. treatment preparation, 2.1.5. controlling ph in solution, 2.1.6. stabilizing water composition during nutrient uptake, 2.2. sample preparation and storage, 2.3. sample analyses, 2.4. modeling approach, 2.4.1. initiating models, 2.4.2. average nitrogen concentration, 2.4.3. monod growth model, 2.4.4. nitrogen uptake, 2.4.5. michaelis–menten uptake model, 2.4.6. shoot-specific cell yield, 3. results and discussion, 3.1. biomass growth and nitrogen depletion, 3.2. monod and michaelis–menten modeling, 3.3. dynamic growth rates, 3.4. visualizing growth models, 3.5. shoot-specific cell yield, 3.6. final considerations, 4. conclusions, author contributions, institutional review board statement, data availability statement, conflicts of interest.
- Halal, W.E.; Davies, O. State of the future, 19.0. World Futures Rev. 2018 , 10 , 95–98. [ Google Scholar ] [ CrossRef ]
- National Academies of Sciences, Engineering, and Medicine. Environmental Engineering for the 21st Century: Addressing Grand Challenges ; National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-47652-2. [ Google Scholar ]
- NAE Grand Challenges for Engineering: 14 Grand Challenges for Engineering in the 21st Century ; National Academy of Engineering: Washington, DC, USA, 2006.
- Enriquez, J.P. Food self-sufficiency: Opportunities and challenges for the current food system. Biomed. J. Sci. Tech. Res. 2020 , 31 , 23984–23989. [ Google Scholar ] [ CrossRef ]
- Kaviti, G.; Asadu, C.; Wiseman, P. Russian war worsens fertilizer crunch, risking food supplies. Associated Press News , 12 April 2022. [ Google Scholar ]
- Zhang, W.; Ma, W.; Ji, Y.; Fan, M.; Oenema, O.; Zhang, F. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in china. Nutr. Cycl. Agroecosyst. 2008 , 80 , 131–144. [ Google Scholar ] [ CrossRef ]
- Steen, I.; Agro, K. Phosphorus availability in the 21st century—Management of a non-renewable resource. Phosphorus Potassium 1998 , 217 , 25–31. [ Google Scholar ]
- Hosseinian, A.; Pettersson, A.; Ylä-Mella, J.; Pongrácz, E. Phosphorus recovery methods from secondary resources, assessment of overall benefits and barriers with focus on the nordic countries. J. Mater. Cycles Waste Manag. 2023 , 25 , 3104–3116. [ Google Scholar ] [ CrossRef ]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009 , 133 , 247–266. [ Google Scholar ] [ CrossRef ]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Micro-Organisms , 14th ed.; Pearson: London, UK, 1992; ISBN 9780321897398. [ Google Scholar ]
- Gellings, C.W.; Parmenter, K.E. Energy efficiency in fertilizer production and use. In Efficient Use and Conservation of Energy: Encyclopedia of Life Support Systems ; EOLSS Publishers: Oxford, UK, 2004. [ Google Scholar ]
- Kool, A.; Marinussen, M.; Blonk, H. LCI Data for the Calculation Tool Feedprint for Greenhouse Gas Emissions of Feed Production and Utilization: GHG Emissions of N, P and K Fertilizer Production ; Blonk Consultants: Gouda, The Netherlands, 2012. [ Google Scholar ]
- FAO. “Energy-Smart” Food for People and Planet ; FAO: Rome, Italy, 2011. [ Google Scholar ]
- Brown, B.D.; Engel, R.; Management, N.; Olson-Rutz, K.; Associate, R. Management to Minimize Nitrogen Fertilizer Volatilization ; Montana State University: Bozeman, MT, USA, 2020. [ Google Scholar ]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011 , 36 , S14–S22. [ Google Scholar ] [ CrossRef ]
- Takaya, N.; Catalan-Sakairi, M.A.B.; Sakaguchi, Y.; Kato, I.; Zhou, Z.; Shoun, H. Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Appl. Environ. Microbiol. 2003 , 69 , 3152–3157. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soussana, J.F.; Allard, V.; Pilegaard, K.; Ambus, P.; Amman, C.; Campbell, C.; Ceschia, E.; Clifton-Brown, J.; Czobel, S.; Domingues, R.; et al. Full accounting of the greenhouse gas (CO 2 , N 2 O, CH 4 ) budget of nine european grassland sites. Agric. Ecosyst. Environ. 2007 , 121 , 121–134. [ Google Scholar ] [ CrossRef ]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.B.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006 , 21 , 192–199. [ Google Scholar ] [ CrossRef ]
- Krauter, C.T.; Potter, C.; Klooster, S. Ammonia Emission Related to Nitrogen Fertilizer Application Practices. In Proceedings of the California Department of Food and Agriculture Fertilizer Education Program Conference, Tulare, CA, USA, 2001. [ Google Scholar ]
- Lammel, G.; Graßl, H. Greenhouse effect of NOX. Environ. Sci. Pollut. Res. 1995 , 2 , 40–45. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Müller, R. The impact of the rise in atmospheric nitrous oxide on stratospheric ozone. Ambio 2021 , 50 , 35–39. [ Google Scholar ] [ CrossRef ]
- Shine, K.P. Radiative forcing of climate change. Space Sci. Rev. 2000 , 94 , 363–373. [ Google Scholar ] [ CrossRef ]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020 , 586 , 248–256. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Berg, M.; Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018 , 28 , 2487–2492.e3. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N 2 O from the nitrogen cycle—Could enzymic regulation hold the key? Trends Biotechnol. 2009 , 27 , 388–397. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Muñoz-Liesa, J.; Rieradevall, J.; Gabarrell, X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? J. Clean. Prod. 2020 , 261 , 121213. [ Google Scholar ] [ CrossRef ]
- Li, W.; Yu, H.; Rittmann, B.E. Reuse water pollutants. Nature 2015 , 528 , 29–31. [ Google Scholar ] [ CrossRef ]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020 , 130 , 109959. [ Google Scholar ] [ CrossRef ]
- Muhaidat, R.; Al-Qudah, K.; Al-Taani, A.A.; AlJammal, S. Assessment of nitrate and nitrite levels in treated wastewater, soil, and vegetable crops at the upper reach of Zarqa river in Jordan. Environ. Monit. Assess. 2019 , 191 , 153. [ Google Scholar ] [ CrossRef ]
- McClintock, S.A.; Sherrard, J.H.; Novak, J.T.; Randall, C.W. Nitrate versus oxygen respiration in the activated sludge process. J. Water Pollut. Control Fed. 1988 , 60 , 342–350. [ Google Scholar ]
- Tomei, M.C.; Carozza, N.A.; Mosca Angelucci, D. Post-aerobic digestion of waste sludge: Performance analysis and modelling of nitrogen fate under alternating aeration. Int. J. Environ. Sci. Technol. 2016 , 13 , 21–30. [ Google Scholar ] [ CrossRef ]
- Lee, E.; Rout, P.R.; Bae, J. The applicability of anaerobically treated domestic wastewater as a nutrient medium in hydroponic lettuce cultivation: Nitrogen toxicity and health risk assessment. Sci. Total Environ. 2021 , 780 , 146482. [ Google Scholar ] [ CrossRef ]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. Biomed. Res. Int. 2015 , 2015 , 404796. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kelemen, D.I. Uptake of Common Pharmaceutical Compounds in Hydroponically Grown Lactuca sativa . Master’s Thesis, University of Connecticut, Storrs, CT, USA, 2018. [ Google Scholar ]
- Altieri, M.A.; Nicholls, C.I. Sustainable Agriculture Reviews ; Springer: Berlin/Heidelberg, Germany, 2012; Volume 11, ISBN 978-94-007-5448-5. [ Google Scholar ]
- Hoque, M.M.; Ajwa, H.A.; Smith, R. Nitrite and ammonium toxicity on lettuce grown under hydroponics. Commun. Soil. Sci. Plant Anal. 2007 , 39 , 207–216. [ Google Scholar ] [ CrossRef ]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006 , 41 , 1667–1673. [ Google Scholar ] [ CrossRef ]
- McCall, D.; Willumsen, J. Effects of nitrate, ammonium and chloride application on the yield and nitrate content of soil-grown lettuce. J. Hortic. Sci. Biotechnol. 1998 , 73 , 698–703. [ Google Scholar ] [ CrossRef ]
- Wang, B.; Shen, Q. Effects of ammonium on the root architecture and nitrate uptake kinetics of two typical lettuce genotypes grown in hydroponic systems. J. Plant Nutr. 2012 , 35 , 1497–1508. [ Google Scholar ] [ CrossRef ]
- Zhang, K.; Burns, I.G.; Turner, M.K. Derivation of a dynamic model of the kinetics of nitrogen uptake throughout the growth of lettuce: Calibration and validation. J. Plant Nutr. 2008 , 31 , 1440–1460. [ Google Scholar ] [ CrossRef ]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011 , 84 , 822–831. [ Google Scholar ] [ CrossRef ]
- Yu, H.Y.; Li, T.X.; Zhang, X.Z. Nutrient budget and soil nutrient status in greenhouse system. Agric. Sci. China 2010 , 9 , 871–879. [ Google Scholar ] [ CrossRef ]
- Epstein, E.; Hagen, C.E. A kinetic study of the absorption of alkali cations by barley roots. Plant Physiol. 1951 , 27 , 457–474. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Griffiths, M.; York, L.M. Targeting root ion uptake kinetics to increase plant productivity and nutrient use efficiency. Plant Physiol. 2020 , 182 , 1854–1868. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barber, S.A.S. Soil Nutrient Bioavailability: A Mechanistic Approach , 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1984. [ Google Scholar ]
- Wheeler, E.F.; Albright, L.D.; Spanswick, R.M.; Walker, L.P.; Langhans, R.W. Nitrate uptake kinetics in lettuce as influenced by light and nitrate nutrition. Trans. ASAE 1998 , 41 , 859–867. [ Google Scholar ] [ CrossRef ]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949 , 3 , 371–394. [ Google Scholar ] [ CrossRef ]
- Strigul, N.; Dette, H.; Melas, V.B. A practical guide for optimal designs of experiments in the Monod model. Environ. Model. Softw. 2009 , 24 , 1019–1026. [ Google Scholar ] [ CrossRef ]
- Grosfils, A.; Vande Wouwer, A.; Bogaerts, P. On a general model structure for macroscopic biological reaction rates. J. Biotechnol. 2007 , 130 , 253–264. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seginer, I.; Buwalda, F.; van Straten, G. Nitrate concentration in greenhouse lettuce: A modeling study. Acta Hortic. 1998 , 456 , 189–197. [ Google Scholar ] [ CrossRef ]
- Seginer, I.; Van Straten, G.; Buwalda, F. Lettuce growth limited by nitrate supply. Acta Hortic. 1999 , 507 , 141–148. [ Google Scholar ] [ CrossRef ]
- Mathieu, J.; Linker, R.; Levine, L.; Albright, L.; Both, A.J.; Spanswick, R.; Wheeler, R.; Wheeler, E.; de Villiers, D.; Langhans, R. Evaluation of the Nicolet model for simulation of short-term hydroponic lettuce growth and nitrate uptake. Biosyst. Eng. 2006 , 95 , 323–337. [ Google Scholar ] [ CrossRef ]
- Ioslovich, I.; Seginer, I.; Baskin, A. Fitting the Nicolet lettuce growth model to plant-spacing experimental data. Biosyst. Eng. 2002 , 83 , 361–371. [ Google Scholar ] [ CrossRef ]
- Juárez-Maldonado, A.; De-Alba-Romenus, K.; Ramírez-Sosa, M.I.M.; Benavides-Mendoza, A.; Robledo-Torres, V. An experimental validation of NICOLET B3 mathematical model for lettuce growth in the southeast region of Coahuila Mexico by dynamic simulation. In Proceedings of the 2010 7th International Conference on Electrical Engineering Computing Science and Automatic Control CCE, Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 128–133. [ Google Scholar ] [ CrossRef ]
- Silberbush, M.; Ben-Asher, J.; Ephrath, J.E. A model for nutrient and water flow and their uptake by plants grown in a soilless culture. Plant Soil. 2005 , 271 , 309–319. [ Google Scholar ] [ CrossRef ]
- Silberbush, M. Nutrients and toxic substances accumulation in the plant and their effect on uptake: Simulation study in hydroponics. Acta Hortic. 2002 , 593 , 235–242. [ Google Scholar ] [ CrossRef ]
- Brechner, M.; Both, A.J. Hydroponic Lettuce Handbook ; Cornell Controlled Environment Agriculture; Cornell University: Ithaca, NY, USA, 1996; Volume 834, pp. 504–509. [ Google Scholar ]
- Modarelli, G.C.; Paradiso, R.; Arena, C.; De Pascale, S.; Van Labeke, M.C. High light intensity from blue-red LEDs enhance photosynthetic performance, plant growth, and optical properties of red lettuce in controlled environment. Horticulturae 2022 , 8 , 114. [ Google Scholar ] [ CrossRef ]
- Mattson, N.S.; Peters, C. A recipe for hydroponic success. Inside Grower. 2014 , Jan , 16–19. [ Google Scholar ]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.A.P.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012 , 84 , 53–61. [ Google Scholar ] [ CrossRef ]
- Safety data sheet for pH up liquid. In General Hydroponics . 2017, Volume 5, pp. 1–10. Available online: https://generalhydroponics.com/resources/ph-up-liquid-safety-data-sheet/ (accessed on 20 June 2024).
- Safety data sheet for pH down liquid. In General Hydroponics . 2017, Volume 5, pp. 1–10. Available online: https://generalhydroponics.com/resources/ph-down-liquid-safety-data-sheet/ (accessed on 20 June 2024).
- Geilfus, C.M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018 , 270 , 114–122. [ Google Scholar ] [ CrossRef ]
- Nissim, W.G.; Masi, E.; Pandolfi, C.; Mancuso, S.; Atzori, G. The response of halophyte ( Tetragonia tetragonioides (pallas) kuntz.) and glycophyte ( Lactuca sativa L.) crops to diluted seawater and NaCl solutions: A comparison between two salinity stress types. Appl. Sci. 2021 , 11 , 6336. [ Google Scholar ] [ CrossRef ]
- Fernandez, D. Phosphorous toxicity and concentration in higher plants. Sci. Hydroponics 2017 . Available online: https://scienceinhydroponics.com/2017/05/phosphorous-toxicity-concentration-higher-plants.html (accessed on 20 June 2024).
- Kim, J.-G.; Kim, M.S. Effects of phosphorus and iron on the phytotoxicity of lettuce ( Lactuca sativa L.) in arsenic-contaminated soil. Ecol. Resilient Infrastruct. 2018 , 5 , 1–10. [ Google Scholar ] [ CrossRef ]
- Uchida, R.; Silva, J.A. Essential nutrients for plant growth: Nutrient functions and deficiency symptoms. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture ; College of Tropical Agriculture and Human Resources: Honolulu, Hawaii, 2000; pp. 31–55. ISBN 978-1-929325-08-5. [ Google Scholar ]
- Kirsten, W.J. Automatic methods for the simultaneous determination of carbon, hydrogen, nitrogen, and sulfur, and for sulfur alone in organic and inorganic materials. Anal. Chem. 1979 , 51 , 1173–1179. [ Google Scholar ] [ CrossRef ]
- USEPA. Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices ; Test methods evaluation solid waste; US Environmental Protection Agency: Washington, DC, USA, 1996.
- Creed, J.T.; Brockhoff, C.A.; Martin, T.D. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-mass Spectrometry ; Revisiom 5.4 EMMC Version; Environmental Monitoring System Laboratory Office Research Development, US Environmental Protection Agency: Washington, DC, USA, 1994; pp. 1–57.
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Mueller, A.; Grisel, O.; Niculae, V.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; et al. API design for machine learning software: Experiences from the Scikit-Learn project. arXiv 2013 . [ Google Scholar ] [ CrossRef ]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine learning in python. J. Mach. Learn. Res. 2011 , 12 , 2825–2830. [ Google Scholar ] [ CrossRef ]
- Karlowsky, S.; Gläser, M.; Henschel, K.; Schwarz, D. Seasonal nitrous oxide emissions from hydroponic tomato and cucumber cultivation in a commercial greenhouse company. Front. Sustain. Food Syst. 2021 , 5 , 626053. [ Google Scholar ] [ CrossRef ]
- Karlowsky, S.; Buchen-Tschiskale, C.; Odasso, L.; Schwarz, D.; Well, R. Sources of nitrous oxide emissions from hydroponic tomato cultivation: Evidence from stable isotope analyses. Front. Microbiol. 2023 , 13 , 1080847. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seo, M.W.; Yang, D.S.; Kays, S.J.; Kim, J.H.; Woo, J.H.; Park, K.W. Effects of nutrient solution electrical conductivity and sulfur, magnesium, and phosphorus concentration on sesquiterpene lactones in hydroponically grown lettuce ( Lactuca sativa L.). Sci. Hortic. 2009 , 122 , 369–374. [ Google Scholar ] [ CrossRef ]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition ; Commonwealth Agricultural Bureaux: Wallingford, UK, 1965. [ Google Scholar ]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950 , 347 . [ Google Scholar ]
- Bollard, E.G.; Butler, G.W. Mineral nutrition of plants. Annu. Rev. Plant Physiol. 1966 , 17 , 77–112. [ Google Scholar ] [ CrossRef ]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the Sixth International Congress on Soilless Culture, ISOSC, Lunteren, The Netherlands, 29 April–5 May 1984; pp. 633–649. [ Google Scholar ]
- Smith, G.S.; Johnston, C.M.; Cornforth, I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983 , 94 , 537–548. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Nitrogen Treatment % | ||||||
|---|---|---|---|---|---|---|
| 200% | 100% | 50% | 25% | 19% | 8% | |
| N | 264.47 | 132.24 | 66.12 | 33.06 | 25.30 | 10.58 |
| P | * | 30.97 | * | 109.86 | 93.94 | 154.05 |
| K | 218.42 | 210.01 | * | * | * | * |
| Ca | * | 82.49 | * | * | * | * |
| S | * | 32.22 | * | * | * | * |
| Mg | * | 24.25 | * | * | * | * |
| Fe | * | 1.26 | * | * | * | * |
| B | * | 162.50 | * | * | * | * |
| Mn | * | 249.04 | * | * | * | * |
| Zn | * | 130.39 | * | * | * | * |
| Cu | * | 23.54 | * | * | * | * |
| Mo | * | 24.09 | * | * | * | * |
| 0 | 2 | 4 | 7 | 9 | 11 | 14 | 16 | 18 | 21 | 23 | 25 | 28 | 30 | 32 | |
| 0 | 1 | 2 | 3 * | 4 | 5 | 6 * | 7 | 8 * | 9 * | 10 * | 11 * | 12 * | 13 * | 14 | |
| 0 | - | - | - | - | - | 1 | - | 2 | 3 | - | 4 | 5 | - | 6 |
| DAT | 11 mg L | 25 mg L | 33 mg L | 66 mg L | 132 mg L | 264 mg L |
|---|---|---|---|---|---|---|
| 0.96 ± 0.33 | 3.20 ± 0.46 | 1.13 ± 0.16 | 2.00 ± 0.51 | 2.61 ± 0.48 | 1.53 ± 0.33 | |
| 0.08 ± 0.03 (8.3%) | 0.24 ± 0.03 (7.5%) | 0.09 ± 0.01 (8.0%) | 0.13 ± 0.02 (6.5%) | 0.21 ± 0.04 (8.0%) | 0.12 ± 0.02 (7.8%) | |
| 1.85 ± 0.77 | 7.04 ± 3.80 | 4.13 ± 0.67 | 9.94 ± 1.56 | 13.07 ± 1.24 | 4.00 ± 0.73 | |
| 0.14 ± 0.06 (7.6%) | 0.48 ± 0.22 (6.8%) | 0.23 ± 0.03 (5.6%) | 0.57 ± 0.07 (5.7%) | 0.88 ± 0.09 (6.7%) | 0.29 ± 0.05 (7.3%) | |
| 4.29 ± 1.05 | 7.37 ± 2.32 | 5.61 ± 1.28 | 11.20 ± 1.81 | 27.63 ± 5.67 | 7.84 ± 1.70 | |
| 0.31 ± 0.07 (7.2%) | 0.46 ± 0.11 (6.2%) | 0.35 ± 0.08 (6.2%) | 0.62 ± 0.14 (5.5%) | 1.74 ± 0.30 (6.3%) | 0.58 ± 0.11 (7.4%) | |
| 9.68 ± 2.39 | 18.46 ± 11.40 | 18.79 ± 3.18 | 28.74 ± 12.30 | 86.81 ± 9.44 | 20.23 ± 4.68 | |
| 0.63 ± 0.14 (6.5%) | 0.98 ± 0.47 (5.3%) | 0.94 ± 0.16 (5.0%) | 1.45 ± 0.51 (5.0%) | 4.55 ± 0.61 (5.2%) | 1.44 ± 0.28 (7.1%) | |
| 15.1 ± 8.48 | 43.07 ± 15.09 | 33.51 ± 6.40 | 71.63 ± 22.52 | 128.71 ± 15.45 | 39.64 ± 9.82 | |
| 1.05 ± 0.45 (7.0%) | 2.31 ± 0.73 (5.4%) | 1.87 ± 0.36 (5.6%) | 3.46 ± 0.92 (4.8%) | 6.38 ± 0.56 (5.0%) | 3.07 ± 0.73 (7.7%) | |
| 32.57 ± 9.22 | 56.62 ± 21.95 | 47.73 ± 7.35 | 92.53 ± 17.97 | 250.73 ± 25.41 | 74.11 ± 10.90 | |
| 1.96 ± 0.47 (6.0%) | 3.00 ± 0.99 (5.3%) | 2.50 ± 0.39 (5.2%) | 4.42 ± 0.54 (4.8%) | 12.10 ± 1.44 (4.8%) | 5.10 ± 0.64 (6.9%) |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Sharkey, A.; Altman, A.; Cohen, A.R.; Groh, T.; Igou, T.K.S.; Ferrarezi, R.S.; Chen, Y. Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture. Agriculture 2024 , 14 , 1358. https://doi.org/10.3390/agriculture14081358
Sharkey A, Altman A, Cohen AR, Groh T, Igou TKS, Ferrarezi RS, Chen Y. Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture. Agriculture . 2024; 14(8):1358. https://doi.org/10.3390/agriculture14081358
Sharkey, Andrew, Asher Altman, Abigail R. Cohen, Teagan Groh, Thomas K. S. Igou, Rhuanito Soranz Ferrarezi, and Yongsheng Chen. 2024. "Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture" Agriculture 14, no. 8: 1358. https://doi.org/10.3390/agriculture14081358
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Depth and microtopography influence microbial biogeochemical processes in a forested peatland
- Research Article
- Published: 12 August 2024
Cite this article

- Ashley D. Keiser ORCID: orcid.org/0000-0002-4381-4974 1 , 2 ,
- Christina L. Davis 2 , 3 ,
- Montana Smith 4 ,
- Sheryl L. Bell 4 ,
- Erik A. Hobbie 5 &
- Kirsten S. Hofmockel 4 , 6
19 Accesses
Explore all metrics
Background and aims
Peat-accumulating wetlands have undulating surfaces of raised areas (hummocks) and depressions (hollows). Hummock-hollow microtopography in relation to the water table influences the distribution of plant species, root density, and microbial community composition, which could in turn alter carbon (C) and nitrogen (N) cycling within peatlands. We used paired hummock and hollow cores from a boreal, forested peatland to assess how microtopography influences peatland microbial function and, in turn, ecosystem C and N cycling.
The peat was analyzed for microbial biomass and potential enzyme activity in 10 cm depth increments relative to the water table, resulting in two increments for hollows and three for hummocks, which has a raised increment above the water table.
Across hummocks and hollows, microbial C and N and fungal biomass generally decreased with depth from the peat surface. In contrast, potential enzyme activity often increased with depth, but this varied within enzyme functional groups according to topography, depth, or both. The potential enzyme activity of C-N degrading peptidases, for example, differed across the five topography × depth increments with the lowest rate in the aerated hummocks. Hummocks compose approximately 66% of the land area at our study site and would therefore underestimate C turnover by an average of 25% if solely used to extrapolate patterns across a forested bog.
Our results suggest that asynchrony in C and N cycling across the undulating surface of forested peatlands impacts our ability to accurately predict biogeochemical cycling across this important ecosystem.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
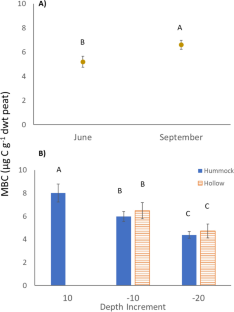
Similar content being viewed by others

Limited effect of drainage on peat properties, porewater chemistry, and peat decomposition proxies in a boreal peatland
Northern peatland carbon dynamics driven by plant growth form — the role of graminoids.

Wetter is Better: Rewetting of Minerotrophic Peatlands Increases Plant Production and Moves Them Towards Carbon Sinks in a Dry Year
Explore related subjects.
- Environmental Chemistry
Data availability
Upon acceptance, the data will be available at: https://mnspruce.ornl.gov/public-data-download , with an associated a doi.
Aerts R (2003) The role of various types of mycorrhizal fungi in nutrient cycling and plant competition. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 117–133
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Change Biol 14:1156–1168
Article Google Scholar
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Article CAS Google Scholar
AminiTabrizi R, Dontsova K, Graf Grachet N, Tfaily MM (2022) Elevated temperatures drive abiotic and biotic degradation of organic matter in a peat bog under oxic conditions. Sci Total Environ 804:150045
Article CAS PubMed Google Scholar
Asemaninejad A, Thorn RG, Lindo Z (2017) Vertical distribution of fungi in hollows and hummocks of boreal peatlands. Fungal Ecol 27:59–68
Bach EM, Hofmockel KS (2014) Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol Biochem 69:54–62
Baldrian P (2014) Distribution of extracellular enzymes in soils: spatial heterogeneity and determining factors at various scales. Soil Sci Soc Am J 78:11–18
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Boelter DH, Verry ES (1977) Peatland and water in the northern lake States. General Technical Report NC-31. USDA Forest Service, St. Paul
Boeraeve M, Kohout P, Ceulemans T, Cajthaml T, Tedersoo L, Jacquemyn H (2022) Changes in the root microbiome of four plant species with different mycorrhizal types across a nitrogen deposition gradient in ombrotrophic bogs. Soil Biol Biochem 169:108673
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Article PubMed Google Scholar
Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, Ellis T, Gerdol R, Hájek M, Hájek T, Iacumin P, Kutnar L, Tahvanainen T, Toberman H (2006) Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc Natl Acad Sci 103:19386–19389
Article CAS PubMed PubMed Central Google Scholar
Bridgham SD, Pastor J, Dewey B, Weltzin JF, Updegraff K (2008) Rapid carbon response of peatlands to climate change. Ecology 89:3041–3048
Brodie E, Edwards S, Clipson N (2003) Soil fungal community structure in a temperate upland grassland soil. FEMS Microbiol Ecol 45:105–114
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Daly AB, Jilling A, Bowles TM, Buchkowski RW, Frey SD, Kallenbach CM, Keiluweit M, Mooshammer M, Schimel JP, Grandy AS (2021) A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry 154:211
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Defrenne CE, Childs J, Fernandez CW, Taggart M, Nettles WR, Allen MF, Hanson PJ, Iversen CM (2021) High-resolution minirhizotrons advance our understanding of root-fungal dynamics in an experimentally warmed peatland. Plants People Planet 3:640–652
Deng Y, Cui X, Hernandez M, Dumont MG (2014) Microbial diversity in hummock and hollow soils of three wetlands on the Qinghai-Tibetan Plateau revealed by 16S rRNA pyrosequencing. PLoS ONE 9:e103115
Article PubMed PubMed Central Google Scholar
Freedman Z, Zak DR (2014) Atmospheric N deposition increases bacterial laccase-like multicopper oxidases: implications for organic matter decay. Appl Environ Microbiol 80:4460–4468
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182
Graham JD, Glenn NF, Spaete LP, Hanson PJ (2020) Characterizing peatland microtopography using gradient and microform-based approaches. Ecosystems 23:1464–1480
Graham EB, Yang F, Bell S, Hofmockel KS (2019) High genetic potential for proteolytic decomposition in Northern Peatland ecosystems. Appl Environ Microbiol 85:e02851–e02818
Guber A, Blagodatskaya E, Kravchenko A (2022) Are enzymes transported in soils by water fluxes? Soil Biol Biochem 168:108633
Hanson PJ, Phillips JR, Nettles WR, Pearson KJ, Hook LA (2020)SPRUCE plot-level water table data assessments for absolute elevtions and height with respect to mean hollows beginning in 2015. In: Oak Ridge National Laboratory, T.S., U.S. Department of Energy (Ed.). https://doi.org/10.25581/spruce.079/1608615
Hanson PJ, Riggs CE, Dorrance C, Nettles WR, Hook LA (2015) SPRUCE environmental monitoring data: 2010–2016. Carbon dioxide information analysis center. Oak Ridge National Laboratory, U.S. Department of Energy. https://doi.org/10.3334/CDIAC/spruce.001
Hargreaves SK, Hofmockel KS (2014) Physiological shifts in the microbial community drive changes in enzyme activity in a perennial agroecosystem. Biogeochemistry 117:67–79
Holden J (2005) Peatland hydrology and carbon release: why small-scale process matters. Philos Trans R Soc A: Math Phys Eng Sci 363:2891–2913
Iversen C, Hanson PJ, Brice DJ, Phillips JR, McFarlane KJ, Hobbie EA, Kolka RK (2014) SPRUCE peat physical and chemical characteristics from experimental plot cores, 2012. Oak Ridge National Laboratory, TES SFA, U.S. Department of Energy, Oak Ridge, Tennessee, U.S.A
Iversen CM, Childs J, Norby RJ, Ontl TA, Kolka RK, Brice DJ, McFarlane KJ, Hanson PJ (2018) Fine-root growth in a forested bog is seasonally dynamic, but shallowly distributed in nutrient-poor peat. Plant Soil 424:123–143
Iversen C, Latimer J, Burnham A, Brice D, Childs J, Vander Stel H (2017) SPRUCE plant-available nutrients assessed with ion-exchange resins in experimental plots, beginning in 2013. Oak Ridge National Laboratory, TES SFA, U.S. Department of Energy, Oak Ridge. https://doi.org/10.3334/CDIAC/spruce.036
Johnson LC, Antoni WHD (1991) Species-controlled sphagnum decay on a South Swedish raised bog. Oikos 61:234–242
Johnson LC, Antoni WHD, Malmer N (1990) Sphagnum macrostructure as an indicator of decay and compaction in peat cores from an ombrotrophic south swedish peat-bog. J Ecol 78:633–647
Juan-Ovejero R, Briones MJI, Öpik M (2020) Fungal diversity in peatlands and its contribution to carbon cycling. Appl Soil Ecol 146:103393
Keiser AD, Smith M, Bell S, Hofmockel KS (2019) Peatland microbial community response to altered climate tempered by nutrient availability. Soil Biol Biochem 137:107561
Kivlin SN, Treseder KK (2014) Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 117:23–37
Kohl L, Laganière J, Edwards KA, Billings SA, Morrill PL, Van Biesen G, Ziegler SE (2015) Distinct fungal and bacterial δ13C signatures as potential drivers of increasing δ13C of soil organic matter with depth. Biogeochemistry 124:13–26
Kotiaho M, Fritze H, Merilä P, Tuomivirta T, Väliranta M, Korhola A, Karofeld E, Tuittila E-S (2013) Actinobacteria community structure in the peat profile of boreal bogs follows a variation in the microtopographical gradient similar to vegetation. Plant Soil 369:103–114
Kuehn KA, Ohsowski BM, Francoeur SN, Neely RK (2011) Contributions of fungi to carbon flow and nutrient cycling from standing dead Typha angustifolia leaf litter in a temperate freshwater marsh. Limnol Oceanogr 56:529–539
Kuznetsova A, Brockhoff P, Christensen R (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Lang AK, Jevon FV, Vietorisz CR, Ayres MP, Hatala Matthes J (2021) Fine roots and mycorrhizal fungi accelerate leaf litter decomposition in a northern hardwood forest regardless of dominant tree mycorrhizal associations. New Phytol 230:316–326
Lenth R (2023). emmeans: estimated marginal means, aka Least-Squares Means. R package version 1.8.4–1. https://rvlenth.github.io/emmeans/
Malhotra A, Brice DJ, Childs J, Graham JD, Hobbie EA, Vander Stel H, Feron SC, Hanson PJ, Iversen CM (2020) Peatland warming strongly increases fine-root growth. Proc Natl Acad Sci 117:17627–17634
Mazziotta A, Granath G, Rydin H, Bengtsson F, Norberg J (2019) Scaling functional traits to ecosystem processes: towards a mechanistic understanding in peat mosses. J Ecol 107:843–859
Minick KJ, Kelley AM, Miao G, Li X, Noormets A, Mitra B, King JS (2019) Microtopography alters hydrology, phenol oxidase activity and nutrient availability in organic soils of a coastal freshwater forested wetland. Wetlands 39:263–273
Näsholm T, Högberg P, Franklin O, Metcalfe D, Keel SG, Campbell C, Hurry V, Linder S, Högberg MN (2013) Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests? New Phytol 198:214–221
Nichols DS (1998) Temperature of upland and peatland soils in a north central Minnesota forest. Can J Soil Sci 78:493–509
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. J Exp Bot 60:1927–1937
Sebestyen SD, Lany NK, Roman DT, Burdick JM, Kyllander RL, Verry ES, Kolka RK (2021) Hydrological and meteorological data from research catchments at the Marcell experimental forest, Minnesota, USA. Hydrol Process 35:e14092
Sinsabaugh R, Findlay S (1995) Microbial production, enzyme activity, and carbon turnover in surface sediments of the Hudson River estuary. Microb Ecol 30:127–141
Smith J, Molina R, Huso MM, Luoma D, McKay D, Castellano M, Lebel T, Valachovic Y (2002) Species richness, abundance, and composition of hypogeous and epigeous ectomycorrhizal fungal sporocarps in young, rotation-age, and old-growth stands of Douglas-fir (Pseudotsuga menziesii) in the Cascade Range of Oregon, USA. Can J Bot 80:186–204
Steinweg JM, Dukes JS, Paul EA, Wallenstein MD (2013) Microbial responses to multi-factor climate change: effects on soil enzymes. Front Microbiol 4:146
Steinweg JM, Kostka JE, Hanson PJ, Schadt CW (2018) Temperature sensitivity of extracellular enzymes differs with peat depth but not with season in an ombrotrophic bog. Soil Biol Biochem 125:244–250
Suriyavirun N, Krichels AH, Kent AD, Yang WH (2019) Microtopographic differences in soil properties and microbial community composition at the field scale. Soil Biol Biochem 131:71–80
Sytiuk A, Céréghino R, Hamard S, Delarue F, Guittet A, Barel JM, Dorrepaal E, Küttim M, Lamentowicz M, Pourrut B, Robroek BJM, Tuittila E-S, Jassey VEJ (2022) Predicting the structure and functions of peatland microbial communities from Sphagnum phylogeny, anatomical and morphological traits and metabolites. J Ecol 110:80–96
Taylor AFS, Fransson PM, Högberg P, Högberg MN, Plamboeck AH (2003) Species level patterns in (13) C and (15) N abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytol 159:757–774
Team RC (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Vance E, Brookes P, Jenkinson D (1987) Microbial biomass measurements in forest soils: the use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol Biochem 19:697–702
Vesala R, Kiheri H, Hobbie EA, van Dijk N, Dise N, Larmola T (2021) Atmospheric nitrogen enrichment changes nutrient stoichiometry and reduces fungal N supply to peatland ericoid mycorrhizal shrubs. Sci Total Environ 794:148737
Wang M, Han Y, Xu Z, Wang S, Jiang M, Wang G (2021) Hummock-hollow microtopography affects soil enzyme activity by creating environmental heterogeneity in the sedge-dominated peatlands of the Changbai Mountains, China. Ecol Indic 121:107187
Wang Y, Li S, Lang X, Huang X, Su J (2022) Effects of microtopography on soil fungal community diversity, composition, and assembly in a subtropical monsoon evergreen broadleaf forest of Southwest China. CATENA 211:106025
Weedon JT, Aerts R, Kowalchuk GA, van Logtestijn R, Andringa D, van Bodegom PM (2013) Temperature sensitivity of peatland C and N cycling: Does substrate supply play a role? Soil Biol Biochem 61:109–120
Weedon JT, Kowalchuk GA, Aerts R, van Hal J, van Logtestijn R, Taş N, Röling WFM, van Bodegom PM (2012) Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob Change Biol 18:138–150
Wiedermann MM, Kane ES, Potvin LR, Lilleskov EA (2017) Interactive plant functional group and water table effects on decomposition and extracellular enzyme activity in Sphagnum peatlands. Soil Biol Biochem 108:1–8
Wu J, Roulet NT, Moore TR, Lafleur P, Humphreys E (2011) Dealing with microtopography of an ombrotrophic bog for simulating ecosystem-level CO2 exchanges. Ecol Model 222:1038–1047
Zhou Y, Qin Y, Liu X, Feng Z, Zhu H, Yao Q (2019) Soil bacterial function associated with stylo (legume) and bahiagrass (grass) is affected more strongly by soil chemical property than by bacterial community composition. Front Microbiol 10:798
Zuo Y, Li J, Zeng H, Wang W (2018) Vertical pattern and its driving factors in soil extracellular enzyme activity and stoichiometry along mountain grassland belts. Biogeochemistry 141:23–39
Download references
Acknowledgements
This research was supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Environmental Systems Science Program, under grant ER 65430 to KSH and Iowa State University, and Iowa State University College of Agriculture and Life Sciences funding to ADK. The SPRUCE experiment is managed by Oak Ridge National Laboratory, which is managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05-00OR22725.
Author information
Authors and affiliations.
Stockbridge School of Agriculture, University of Massachusetts Amherst, Amherst, MA, USA
Ashley D. Keiser
Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, IA, USA
Ashley D. Keiser & Christina L. Davis
Department of Natural Resources and Sciences, McGill University, Montreal, QC, Canada
Christina L. Davis
Earth and Biological Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA, USA
Montana Smith, Sheryl L. Bell & Kirsten S. Hofmockel
Earth Systems Research Center, University of New Hampshire, Durham, NH, USA
Erik A. Hobbie
Department of Agronomy, Iowa State University, Ames, IA, USA
Kirsten S. Hofmockel
You can also search for this author in PubMed Google Scholar
Contributions
Study conception and design was led by Kirsten Hofmockel. Material preparation and data collection were performed by Kirsten Hofmockel, Montana Smith, Sheryl Bell, and Erik Hobbie. Data analysis was performed by Christina Davis and Ashley Keiser. The first draft of the manuscript was written by Ashley Keiser and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ashley D. Keiser .
Ethics declarations
Competing interests.
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Yolima Carrillo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 587 kb)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Keiser, A.D., Davis, C.L., Smith, M. et al. Depth and microtopography influence microbial biogeochemical processes in a forested peatland. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06895-1
Download citation
Received : 25 January 2024
Accepted : 05 August 2024
Published : 12 August 2024
DOI : https://doi.org/10.1007/s11104-024-06895-1

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Microtopography
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Original Article
- Open access
- Published: 25 July 2017
Nematode grazing promotes bacterial community dynamics in soil at the aggregate level
- Yuji Jiang 1 ,
- Manqiang Liu ORCID: orcid.org/0000-0001-6654-7795 2 ,
- Jiabao Zhang 1 ,
- Yan Chen 1 ,
- Xiaoyun Chen 2 ,
- Lijun Chen 1 ,
- Huixin Li 2 ,
- Xue-Xian Zhang 3 &
The ISME Journal volume 11 , pages 2705–2717 ( 2017 ) Cite this article
10k Accesses
188 Citations
11 Altmetric
Metrics details
- Microbial ecology
Nematode predation has important roles in determining bacterial community composition and dynamics, but the extent of the effects remains largely rudimentary, particularly in natural environment settings. Here, we investigated the complex microbial–microfaunal interactions in the rhizosphere of maize grown in red soils, which were derived from four long-term fertilization regimes. Root-free rhizosphere soil samples were separated into three aggregate fractions whereby the abundance and community composition were examined for nematode and total bacterial communities. A functional group of alkaline phosphomonoesterase (ALP) producing bacteria was included to test the hypothesis that nematode grazing may significantly affect specific bacteria-mediated ecological functions, that is, organic phosphate cycling in soil. Results of correlation analysis, structural equation modeling and interaction networks combined with laboratory microcosm experiments consistently indicated that bacterivorous nematodes enhanced bacterial diversity, and the abundance of bacterivores was positively correlated with bacterial biomass, including ALP-producing bacterial abundance. Significantly, such effects were more pronounced in large macroaggregates than in microaggregates. There was a positive correlation between the most dominant bacterivores Protorhabditis and the ALP-producing keystone 'species' Mesorhizobium . Taken together, these findings implicate important roles of nematodes in stimulating bacterial dynamics in a spatially dependent manner.
Similar content being viewed by others

Conventional and organic soil management as divergent drivers of resident and active fractions of major soil food web constituents
Response of soil nematode community structure and metabolic footprint to nitrogen addition in alfalfa fields on the Loess Plateau
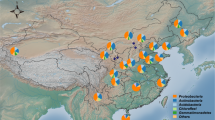
Soil bacterial diversity correlates with precipitation and soil pH in long-term maize cropping systems
Introduction.
Resource competition and predation are the two major driving forces underlying the dynamic changes of species composition in the biological community ( Chesson and Kuang, 2008 ). For microorganisms inhabiting soil, the importance of resource competition has been well documented, particularly with the improved accessibility of next-generation sequencing and stable isotope techniques ( Bulgarelli et al., 2013 ). Although the importance of predation by microfauna has long been recognized, the potential effects of predation remain poorly defined. Few studies have addressed the complex microbial–microfaunal interactions in open-field environments ( Neher, 2010 ). For example, nematodes can stimulate microbial activity, resulting in either an increase or a decrease of microbial biomass in microcosm experiments ( Trap et al., 2016 ). The grazing-induced influences on microbial abundance vary according to pore structure and distribution of the accessible resources such as soil organic matter and plant roots ( Rønn et al., 2012 ).
Soils have a complex hierarchical structure including pore distribution and aggregates. Soil aggregates provide spatially heterogeneous habitats for microorganisms, which vary in nutrient availability, water potential and oxygen concentration as well as predation pressure ( Ranjard, Richaume, 2001 ; Jiang et al., 2013 ). Aggregate fractions are assembled by organic matter and mineral particles ( Tisdall and Oades, 1982 ). Macroaggregates normally contain more labile substrates predominantly derived from plant residues ( Bronick and Lal, 2005 ), and harbor higher amounts of fungal biomass than microaggregates (MAs, Rillig and Mummey, 2006 ). In contrast, MAs are characterized by the highest concentration of stable organic carbon, and more importantly, they provide a protective microenvironment for microbial growth ( Six et al., 2000 ). The relative small pore sizes make MAs inaccessible to large-sized bacterial-feeding nematodes (typically 30–90 μm in diameter; Quénéhervé and Chotte, 1996 ). Thus, it is crucial to understand the predator–prey interactions at the aggregate level ( Ettema and Wardle, 2002 ).
Of particular significance is the rhizosphere soil as it functions as the critical interface for resource exchange between plants and soil. Compared with bulk soil, the rhizosphere contains a large amount of small organic compounds excreted from the roots of living plants, supporting high levels of microbial activity. Microorganisms are integral to the cycling of nutritional elements such as nitrogen and phosphorus ( van der Heijden et al., 2008 ), and bacterial-feeding nematodes predation releases nutrients sequestered in bacterial biomass in the rhizosphere niche ( Bonkowski et al., 2009 ). However, nematodes normally have special food preferences, and bacteria were not equally susceptible to predation by nematodes. Selective grazing by nematodes can alter the bacterial community composition ( Djigal et al., 2004 ). This leads to an important but unexplored hypothesis that nematode grazing affects total bacterial community and specific functional groups such as phosphorus (P) cycling in different manners.
Microbial–microfaunal interactions via the microbial loop determine the rate of P cycling in the rhizosphere ( Bonkowski, 2004 ). However, the specific effects of nematode grazing on phosphate solubilizing microbial community remain poorly understood. Phosphorus is one of the most limiting nutrients in agricultural soils ( Tabatabai, 1994 ). Predominant enzymes involved in organic P mineralization are alkaline phosphomonoesterases (ALPs, EC 3.1.3.1) and acid phosphomonoesterases (ACPs, EC 3.1.3.2) ( Nannipieri et al., 2011 ; Chang et al., 2015 ). ALPs are mostly of bacterial origin, whereas ACPs are mainly excreted by plant roots and fungi in the rhizosphere ( Tabatabai, 1994 ; Spohn and Kuzyakov, 2013 ). Significantly, ALP-producing bacterial community can be quantitatively analyzed using phoD gene as a molecular marker ( Sakurai et al., 2008 ). The phoD gene abundance is positively correlated with ALP activity as revealed by field studies with bulk soil ( Tan et al., 2013 ; Fraser et al., 2015a , b ).
Here, we investigated the reciprocal interactions between nematodes and bacteria in rhizosphere soil at the aggregate level, with an additional specific focus on ALP-producing bacteria. To this end, we performed a 13-year field experiment with red soils (Acrisol) under four fertilization regimes. Soil samples were taken from the rhizosphere of maize, and then separated into three aggregate size fractions for physiochemical and microbiological analyses. The nematode assemblages were quantitatively assessed under microscope, whereas the abundance and composition of bacterial community were examined using phospholipid fatty acid analysis and Illumina sequencing of 16S rRNA gene, respectively. Next, the abundance and composition of ALP-producing bacterial community were estimated using quantitative polymerase chain reaction (PCR) and Illumina sequencing of phoD gene. We observed significantly positive influences of nematodes on bacterial abundance and activity, which were subsequently confirmed via pot experiments under well-controlled laboratory conditions. Our findings provided insights into the microbial–microfaunal interactions in the rhizosphere at the soil aggregate level.
Materials and methods
Site description and design.
The long-term fertilization experiment was conducted at the National Agro-Ecosystem Observation and Research Station in a subtropical humid monsoon climate region China (Yingtan, 28°15′N, 116°55′E) with an annual average temperature 17.6 °C and precipitation 1795 mm. The soil is an acid loamy clay-derived Quaternary red clay (Udic Ferralsols in the Chinese Soil Taxonomy and Ferric Acrisols in the FAO classification system).
Twelve concrete lysimeters, 2 m wide × 2 m long × 1.5 m deep, were used in the manure experiment since 2002. Four pig manure rates were compared in a completely randomized design with three replicates: (1) no manure (M0); (2) low manure with 150 kg N ha −1 y −1 (M1); (3) high manure with 600 kg N ha −1 y −1 (M2); and (4) high manure with 600 kg N ha −1 y −1 and lime (M3; Ca(OH) 2 applied once every 3 years at 3000 kg ha −1 ). The pig manure contained an average total carbon of 386.5 g kg −1 , total nitrogen of 36.2 g kg −1 and total phosphorus of 21.6 g kg −1 on a dry matter basis. The monoculture maize ( Zea mays L.), cultivar No.11 from Denghai, was planted annually in April and harvested in July from 2002 to 2014. There were no tillage and management measures with the exception of manual weeding.
Soil sampling and aggregate fractionation
Soil sampling was conducted in late July 2014 after 13 years of fertilization. Rhizosphere soils were collected from each plot at a depth of 0−15 cm, and then were placed on ice and immediately transported to the laboratory. After shaking off the loosely adhering soil, the tightly adhering rhizosphere soil was collected with a brush, passed through a 4 mm sieve. Next, 100 g root-free soil was manually fractionated through a series of two sieves (2000 μm and 250 μm) into three aggregate sizes: large macroaggregates (>2000 μm; LMA), small macroaggregates (250–2000 μm; SMA) and MA (<250 μm; Jiang et al., 2014 ). Each aggregate fraction was homogenized for chemical and biological analyses. Standard methods were used to characterize soil chemical properties and phosphomonoesterase activities ( Supplementary Appendix ).
Characterization of total bacteria and ALP-producing bacteria
A modified method of phospholipid fatty acids (PLFAs) analysis was used to measure soil bacterial biomass, which is expressed as nanomoles of PLFA per gram of dry soil ( Frostegård and Bååth, 1996 ). DNA was extracted from 0.5 g fresh soil using the Ultraclean Soil DNA Isolation Kit (Mo Bio Laboratories, Inc., Carlsbad, CA, USA) following the manufacturer’s instructions. Real-time quantitative PCR analysis of phoD gene was performed with primers ALPS-F730 and ALPS-R1101 ( Sakurai et al., 2008 ). For high-throughput sequencing at the Illumina MiSeq platform, the V3–V4 region of 16S rRNA gene and phoD gene were amplified separately using primers 338 F/806 R and ALPS-F730/ALPS-R1101, respectively. Raw sequences were quality screened and trimmed, including quality trimming, de-multiplexing, taxonomic assignments, chimera detection, and screening for frame shifts. Thereafter, the 16S rDNA and phoD sequences were subjected to a similarity search against the Ribosomal Database Project database and the GenBank non-redundant nucleotide database, respectively. Finally, the sequence reads from each sample were clustered to operational taxonomic units (OTUs) at 97% similarity. Alpha diversity metrics and community relatedness were calculated at the same sequencing depth. The 16S rDNA and phoD sequences are available at the NCBI Sequence Read Archive under accession number SRP090422 and SRP044878, respectively (see details in Supplementary Appendix ).
Nematode faunal composition
Nematodes were extracted using a modified Baermann funnel method ( Barker, 1985 ), and visually examined with an inverted compound microscope. At least 100 nematodes were identified to the genus level for each sample. Nematodes were divided into four trophic groups: bacterivores (Ba), fungivores (Fu), plant parasites (Pp) and omnivores-predators, characterized by known feeding habitats or stoma and esophageal morphology ( Yeates et al., 1993 ). The guilds were characterized on the colonizer-persister ( c − p ) scale (1−5) as previously described ( Bongers and Bongers, 1998 ).
Microcosm experiment
Rhizosphere soils were sterilized by acute gamma irradiation at 40 kGy doses ( Buchan et al., 2012 ). Bacterial suspensions of fresh soils were prepared by passing through 1 μm pore-size Millipore filters (Millipore, Bedford, MA, USA) whereby nematodes and other small eukaryotes were eliminated. The dominant bacterivorous nematode, Protorhabditis spp., isolated from the experimental site was cultivated in nematode growth medium at 28 °C by feeding on Escherichia coli . Before use, nematodes were washed five times with sterile distilled water to minimize the effects of E. coli .
To set up the microcosms, 50, 150, 500 and 600 individuals were introduced into 100 g soil per pot for soils obtained from the M0, M1, M2 and M3 treatments, respectively. Nematode-free control was set up in triplicate to ensure no nematode contamination. Microcosms were incubated in the dark at 28 °C, with soil moisture being maintained at 25% (w/w). Soils were destructively sampled in 0, 3, 7, 14 and 21 days after inoculation, and then separated into three aggregate fractions for analysis of nematodes, ALP-producing bacteria, ALP and ACP activities.
Statistical analyses
The statistical procedures, including Pearson’s correlation analysis, were conducted by SPSS statistical software (SPSS Inc., Chicago, IL, USA). The aggregated boosted trees analysis was carried out to evaluate the effects of different factors on ALP-producing bacterial abundance and activity ( De'Ath, 2007 ). The canonical analysis of principal coordinates was performed to assess the influence of different experimental factors on beta diversity ( Anderson and Willis, 2003 ). Driving factors for nematodes and ALP-producing bacterial community composition were quantitatively evaluated using the permutational multivariate analysis of variance (ANOVA; Anderson, 2001 ). Structural equation modeling was used to understand how soil chemical properties altered nematodes and bacterial community in three aggregate fractions ( Byrne, 2010 ). Interaction networks were constructed by calculating the pairwise Spearman’s rank correlations (see the details in Supplementary Appendix ).
Soil physiochemical properties at the aggregate level
Rhizosphere soils from the four fertilization treatments were separated into three aggregate fractions: LMA (>2 mm), SMA (0.25–2 mm) and MAs (<0.25 mm). Results of two-way ANOVA revealed significant differences in soil physiochemical properties among treatments ( F (3,32) =64.59–1222.85, P <0.001) and aggregate fractions ( F (2,33) =3.73–21.15, P <0.05). High levels of manure treatments (M2 and M3) contained higher proportions of LMA fractions compared with low manure treatment (M1) and the control (M0; Supplementary Figure 1a ). Soil pH was significantly elevated by high manure application ( Supplementary Figure 1b ). The MA fraction tended to have higher nutritional substrates than the LMA and SMA fractions in terms of soil organic carbon ( Supplementary Figure 1c ), total nitrogen ( Supplementary Figure 1d ) and phosphate contents ( Supplementary Figures 2a and b ). The similar trend was found for soil enzymatic activities of ACP and ALP, respectively ( Supplementary Figures 2c and d ). Both ACP and ALP activities showed significant correlations with total phosphate ( r =0.637 and r =0.953, respectively) and available phosphate ( r =0.607 and r =0.958, respectively) at the level of P <0.001.
Characterizing the bacterial community in soil aggregates
Soil aggregate samples were subjected to PLFA analysis for bacterial biomass, and Illumina sequencing of 16S rRNA gene for the diversity and composition of bacterial community. The results indicated significant differences in fertilization treatments and aggregate fractions ( Figure 1 , P <0.05). Higher manure application resulted in higher bacterial biomass and diversity, showing a general trend of M3≈M2>M1>M0 ( Figures 1a–c ). The MA fraction possessed the highest bacterial biomass and diversity than the LMA fraction, with the SMA as the intermediates ( Figures 1a–c ).
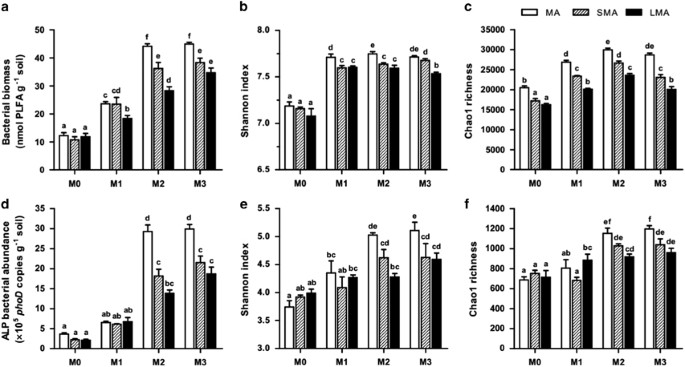
Fertilization and aggregate fractions alter rhizosphere bacterial biomass and diversity. The biomass ( a ), diversity ( b ) and richness ( c ) of total bacterial community were examined together with the abundance ( d ), diversity ( e ) and richness ( f ) of the ALP-producing bacteria in the rhizosphere. Calculation of diversity and richness is based on OTU tables rarified to the same sequencing depth. Error bars represent standard errors of three replicates. Bars with the different letter (shown above each) are significantly different ( P <0.05) by Tukey’s HSD test. ALP, alkaline phosphomonoesterase. M0, no manure; M1, low manure; M2, high manure; M3, high manure plus lime. HSD, honest significant difference; LMA, large macroaggregate; MA, microaggregate; SMA, small macroaggregate.
The bacterial communities were dominated by Chloroflexi (19.6%), Actinobacteria (19.0%), Alphaproteobacteria (11.3%), Firmicutes (10.3%), Acidobacteria (8.3%), Deltaproteobacteria (6.7%) and Gammaproteobacteria (5.1%; Figure 2a ). In addition, Betaproteobacteria, Bacteroidetes, Cyanobacteria, Gemmatimonadetes, Planctomycetes and Verrucomicrobia were present at lower abundances, accounting for 14.7% of all sequences ( Figure 2a ). Bray-Curtis distances derived from a canonical analysis of principal coordinates were used to compare bacterial community composition between three aggregate fractions. Bacterial community composition in the MA fraction was well separated from those of the LMA and SMA fractions, mainly because of the higher abundance of Chloroflexi and Cyanobacteria but the lower abundance of Alphaproteobacteria , Gammaproteobacteria and Actinobacteria ( Figure 3a ). Finally, permutational multivariate ANOVA of bacterial community composition showed that 75.5% of variations could be explained by fertilization (59.3%) and aggregate fractions (14.2%; Supplementary Table 1 ).
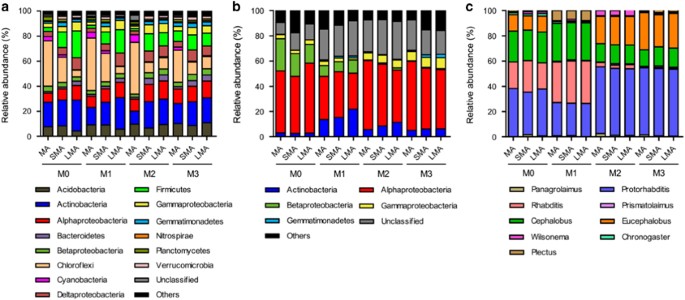
Taxonomic compositions of bacterial community and bacterivores assemblages. The abundances of total bacterial ( a ) and ALP-producing bacterial ( b ) communities are based on the proportional frequencies of 16S rRNA- and phoD- like sequences. The abundance of bacterivores ( c ) is calculated in bacterivorous guilds. ALP, alkaline phosphomonoesterase. M0, no manure; M1, low manure; M2, high manure; M3, high manure plus lime. LMA, large macroaggregate; MA, microaggregate; SMA, small macroaggregate.
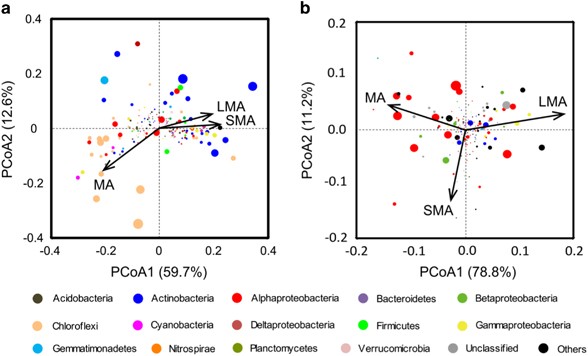
Aggregate fractions alter the bacterial community composition. The dominant OTU (relative abundance >0.1%) scores in ( a ) total bacterial and ( b ) ALP-producing bacterial community by principal coordinate analysis, which are constrained by aggregate fractions and based on Bray-Curtis distances among all the samples. The arrows point to the centroid of the constrained factor. Circle sizes correspond to the abundance of total bacterial and ALP-producing bacterial OTUs, and colors are assigned to different phyla/classes. ALP, alkaline phosphomonoesterase. M0, no manure; M1, low manure; M2, high manure; M3, high manure plus lime. LMA, large macroaggregate; MA, microaggregate; SMA, small macroaggregate.
Characterizing the ALP-producing bacterial community in soil aggregates
The abundance of ALP-producing bacteria was expressed as the phoD gene copy number per gram of dry soil as estimated by quantitative PCR analysis ( Figure 1d ). It followed a similar trend as total bacterial biomass, with relatively higher abundance under manure treatments (M3≈M2>M1>M0). There were significantly more ALP-producing bacteria in the MA fraction compared with the LMA and SMA fractions under M2 and M3 treatments. There was a significant positive correlation between ALP-producing bacterial abundance and ALP activity ( r =0.965, P <0.001; Supplementary Figure 3 ). Both were most significantly influenced by soil pH, as indicted by aggregated boosted tree analysis ( Supplementary Figure 4 ).
The diversity and composition of ALP-producing bacterial community were examined using Illumina sequencing of the phoD gene. Similar trends of the Shannon index and Chao1 richness were found for total bacteria and the specific functional group of ALP-producing bacteria: M3≈M2>M1>M0 for the effects of manure addition, and MA>SMA>LMA for variations among soil aggregates ( Figure 1 ). The ALP-producing bacterial community was dominated by Alphaproteobacteria (45.4%), Actinobacteria (8.5%), Betaproteobacteria (7.5%) and Gammaproteobacteria (5.0%; Figure 2b ). The ALP-producing bacterial communities from the three aggregate fractions were well separated by PCoA1 alone (78.8%, Figure 3b ). There were significant differences among soil aggregates in regards to the abundance of ALP-producing Alphaproteobacteria ( P =0.033) and Gammaproteobacteria ( P =0.001; Supplementary Figure 5 ). The permutational multivariate ANOVA showed that the variations of ALP-producing bacterial community structure under fertilization treatments (61.5%) were much bigger than those among aggregate fractions (9.7%; Supplementary Table 1 ). This has been further demonstrated by hierarchical clustering analysis of dominant OTUs based on their co-occurrence ( Supplementary Figure 6 ).
Investigating the nematode assemblages in soil aggregates
A total of 26 nematode genera were identified including nine bacterivores, five fungivores, four plant parasites and eight omnivores-predators ( Supplementary Table 2 ). On average, bacterivores (46.6%) and plant parasites (30.5%) were the two most abundant trophic groups. The five dominant genera of nematodes were Protorhabditis , Cephalobus , Eucephalobus, Pratylenchus and Mesodorylaimus , cumulatively representing over 70% of all nematodes identified ( Supplementary Table 2 ). The average number of total nematodes increased with increasing aggregate size, such that the LMA fraction had a significantly higher number than the SMA and MA fractions ( Supplementary Table 2 , P <0.05). The bacterivores, plant parasites and omnivores-predators, particularly the dominant genus within each guild ( Protorhabditis , Pratylenchus and Mesodorylaimus , respectively), appeared to exhibit a general trend of LMA>SMA>MA, which was similar to that of the number of nematodes in total ( Figure 2c , Supplementary Table 2 ). The permutational multivariate ANOVA showed that the nematode community structure was influenced by fertilization treatments (56.7%) and aggregate fractions (11.0%), which collectively explained 67.7% of the total variations ( Supplementary Table 1 ).
Ecological interactions between nematodes and bacteria in soil aggregates
The abundance of bacterivorous nematodes were positively correlated with the two different measures of bacterial abundance, that is, total bacterial biomass ( r =0.798, P <0.001) and ALP-producing bacterial abundance ( r =0.783, P <0.001), and ALP activity ( r =0.843, P <0.001), rather than ACP activity ( r =0.318, P =0.059; Supplementary Figure 3 , Supplementary Table 3 ). Intriguingly, the obtained data revealed positive correlations between bacterivore abundance and bacterial diversity: total bacterial diversity ( r =0.655, P <0.001) and richness ( r =0.430, P <0.001), as well as ALP-producing bacterial diversity ( r =0.675, P <0.001) and richness ( r =0.715, P <0.001; Supplementary Table 3 ). Furthermore, the community composition of total bacteria and ALP-producing bacteria were significantly affected by nematodes (21.0 and 22.3%), with the largest contribution from bacterivores (12.9 and 13.5%) ( Supplementary Table 4 ).
The structural equation model was used to assess the effects of soil properties and nematodes on the bacterial community in the three aggregate fractions. Soil organic carbon produced the strongest effects on total bacterial biomass and community composition, while total bacterial diversity was primarily determined by soil pH ( Figure 4 ). More importantly, bacterivores had positive effects on bacterial diversity (path coefficient: 0.35, P =0.017) and community composition (path coefficient: 0.48, P =0.003) in the LMA fraction ( Figure 4c ). For ALP-producing bacteria, soil pH was one of the most important causal factors determining ALP-producing bacterial abundance and ALP activity ( Figures 4d−f ). Bacterivores exerted more prominent contribution to ALP-producing bacterial abundance in the LMA fraction (path coefficient: 0.57, P <0.001) than in the SMA (path coefficient: 0.29, P =0.026) and MA (path coefficient: 0.32, P =0.022) fractions. Similar to total bacteria, the ALP-producing bacterial community composition was positively affected by bacterivores in the LMA fractions (path coefficient: 0.44, P <0.001; Figure 4f ).
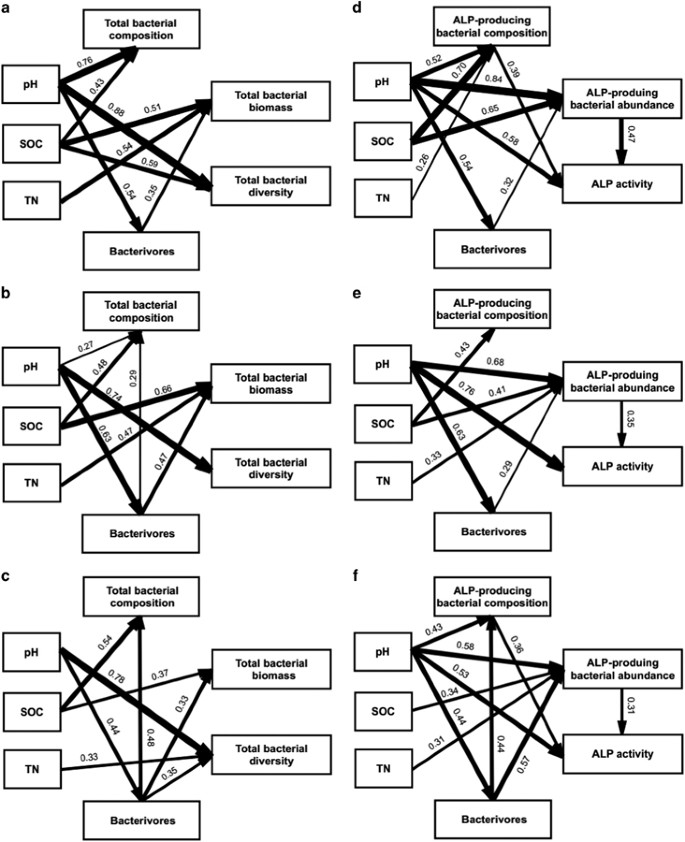
The effects of soil properties and nematodes on bacterial community as estimated using the structural equation model. For total bacteria, ( a ) microaggregates, χ 2 =7.969, GIF=0.977, P =0.527, AIC=37.692, RMSEA=0.001; ( b ) small macroaggregates, χ 2 =4.341, GIF=0.956, P =0.332, AIC=48.701, RMSEA=0.003; and ( c ) large macroaggregates, χ 2 =5.058, GIF=0.951, P =0.561, AIC=59.408, RMSEA=0.008. For ALP-producing bacteria, ( d ) microaggregates, χ 2 =5.949, GIF=0.957, P =0.486, AIC=57.496, RMSEA<0.001; ( e ) small macroaggregates, χ 2 =4.219, GIF=0.958, P =0.183, AIC=50.190, RMSEA=0.007; and ( f ) large macroaggregates, χ 2 =4.115, GIF=0.961, P =0.245, AIC=69.151, RMSEA<0.001. The first principal coordinates (PCoA1 explained 59.7 and 78.8% of the variations, see Figure 3 ) are used to represent the composition of total bacterial and ALP-bacterial community. The width of black arrows indicates the strength of significant standardized path coefficients ( P <0.05). ALP, alkaline phosphomonoesterase; SOC, soil organic carbon; TN, total nitrogen.
Interaction network between nematodes and bacterial community
We sought to determine the co-occurrence patterns of nematodes and bacteria using network analysis based on strong and significant correlations. The calculated modularity index was larger than 0.4 ( Table 1 ), indicating a typical module structure ( Newman, 2006 ). Overall, aggregate fractions showed a remarkable effect on association networks of nematodes and bacteria, as well as ALP-producing bacteria. For total bacteria and ALP-producing bacteria, the values of average path length, average clustering coefficient ( avgCC ) and modularity in these empirical networks were higher than those of their respective identically sized Erdös–Réyni random networks ( Table 1 ). Furthermore, average connectivity ( avgK ) and modularity was greater in the LMA than in the SMA and MA networks, whereas average path length followed the opposite trend.
The co-occurrence patterns between bacterivores and ALP-producing bacteria were further compared across three aggregate fractions. Notably, there were more positive than negative correlations in all networks, regardless of aggregate fractions ( Table 1 ). Bacterivores were more closely (for example, more abundant nodes) correlated with ALP-producing bacteria in the LMA than in the SMA and MA fractions ( Figure 5 , Table 1 ). In particular, the dominant bacterivores Protorhabditis (degree=15) showed stronger positive correlations with ALP-producing bacteria in the LMA network ( Figure 5 ). Topologically, the individual nodes played different roles in the networks according to two properties: the within-module degree Z and among-module degree P . The genus Mesorhizobium (class Alphaproteobacteria ) was categorized as the module hub for all three networks ( Figure 5 , Table 2 ). Notably, bacterivores Protorhabditis showed strong positive correlations with module hubs OTU2517 ( r =0.861, P =0.006), OTU1444 ( r =0.822, P =0.009) and OTU1352 ( r =0.958, P <0.001), and explained more than one-fourth of variations in the abundance of three module hubs ( Supplementary Table 5 ).
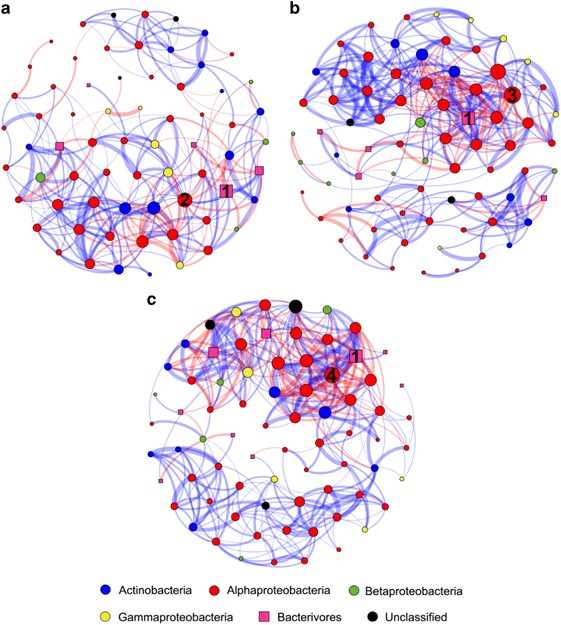
Interaction networks between bacterivorous nematodes and ALP-producing bacterial communities. A connection stands for a strong (Spearman’s ρ >0.8) and significant ( P <0.01) correlation for the MA ( a ), SMA ( b ) and LMA ( c ) fractions. The co-occurring networks are colored by phylum/class. For each panel, the size of each node is proportional to the number of connections (that is, degree), and the thickness of each connection between two nodes (that is, edge) is proportional to the value of Spearman’s correlation coefficients. A blue edge indicates a positive interaction between two individual nodes, while a red edge indicates a negative interaction. The numbers inside the nodes are as follows: (1) the dominant bacterivores Protorhabditis , (2) the module hub OTU2517, (3) the module hub OTU1444 and (4) the module hub OTU1352. ALP, alkaline phosphomonoesterase; LMA, large macroaggregate; MA, microaggregate; SMA, small macroaggregate.
Verifying the nematodes–bacteria interactions by soil microcosm experiments
Having found positive effects of nematode grazing on ALP-producing bacterial abundance and activity in open-field environments, we proceeded to demonstrate this in the microcosm under well-controlled laboratory conditions. We applied the natural microbial community with and without bacterivorous nematodes to pre-sterilized soils. Dynamic changes of bacterivores, ALP-producing bacteria and ALP activity were monitored over a period of 21 days. Parallel to our expectation, there were remarkable increases in nematodes, ALP-producing bacteria abundance and ALP activity over time ( P <0.001), particularly under M2 and M3 treatments. Specifically, the abundance of Protorhabditis was approximately 25% higher in the LMA fraction than that in the MA fraction ( Figure 6 ). Protorhabditis produced significant effects on ALP-producing bacterial abundance and ALP activity under M2 and M3 treatments compared to under M0 and M1 treatments ( Supplementary Figure 7 ). After 14 days’ incubation, ALP-producing bacterial abundance and ALP activity were elevated by 23.1 and 12.3% with bacterivores addition under the M2 treatment, and increased by 30.3 and 14.1% under the M3 treatment, respectively ( Figure 6 ). Significantly, the effects of Protorhabditis grazing were two to three times larger in the LMA fraction relative to the MA fraction under M2 and M3 treatments ( Figure 6 ).
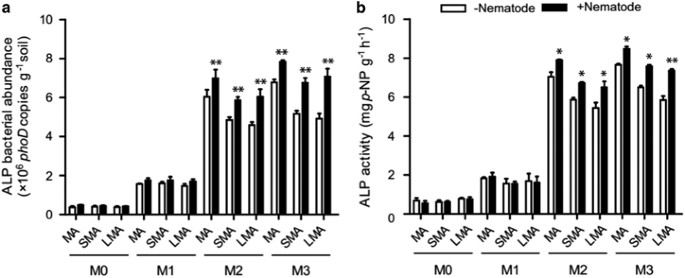
Microcosm experiment showing the effects of nematode grazing on ALP-producing bacterial abuncance ( a ) and ALP activity ( b ). Data are means and standard errors of three replicates with (+Nematode) and without (−Nematode) the inoculation of Protorhabditis in 14 days. Asterisks over the time indicate a significant difference (** P <0.01; * P <0.05). M0, no manure; M1, low manure; M2, high manure; M3, high manure plus lime. ALP, alkaline phosphomonoesterase; MA, microaggregate; LMA, large macroaggregate; SMA, small macroaggregate.
Predator–prey interactions are building blocks of food webs, whose stability requires a negative feedback loop. Specifically, an increase of predator abundance causes declines in prey populations, which in turn prevents further increase of the predator population ( Djigal et al., 2004 ). However, a great number of theoretical and empirical studies show that predators can also produce positive effects on their prey, and vice versa ( Ingham et al., 1985 ; Brown et al., 2004 ; Fu et al., 2005 ). The underlying mechanisms include enhanced nutrient mineralization ( Diehl et al., 2000 ), disposal to new niches for colonization ( Ingham et al., 1985 ), as well as the emergence of novel physical and behavioral prey refuges ( Cressman and Garay, 2009 ). The net influence between predator and prey is dependent on a combination of both positive and negative effects, and it is likely subject to temporal and spatial dynamic changes. Significantly, when the predator–prey interactions are extended from simple pairs to community levels, such as the bacterivores–bacteria relationships in soil, it remains possible that the two communities may display no ecological correlations, likely owing to the effects of species compensation. In heterogeneous soil environments, the complex bacterivores–bacteria interaction networks form the basis of the heterotrophic eukaryotic food web, ensuring energy flows through the bacterial energy channel to higher trophic levels ( Bonkowski et al., 2009 ). It is thus important to understand the ecological relationships between the bacterial community and their predatory bacterivores in various soils.
Here, we found that bacterivores significantly enhanced bacterial abundance in the maize rhizosphere across four fertilization treatments and three aggregate fractions. This result was initially surprising, as a recent meta-analysis revealed that bacterivores caused a 16 and 17% reduction in soil microbial biomass and bacterial abundance, respectively ( Trap et al., 2016 ). However, our findings were consistent with previous results from microcosm studies, showing that moderate grazing of microbivores on microflora could stimulate microbial growth ( Fu et al., 2005 ). A plausible explanation is that certain bacterivores feed on senescent bacterial cells, and consequently stimulate nutrient cycling ( Ingham et al., 1985 ). In addition, many bacteria reside at the body surfaces or in the digestive systems of nematodes ( Neher, 2010 ). The movement of nematodes can help disperse bacteria to new niches for colonization in heterogeneous soil environments.
With regard to the effects of bacterivores on bacterial community, we observed positive correlations between bacterivores abundance and bacterial diversity in terms of both Shannon index and Chao1 richness. Moreover, bacterivores caused significant changes in bacterial community composition. These data clearly indicated that nematode predation had significant roles in driving dynamic changes of the bacterial community. Bacterivorous nematodes could potentially promote bacterial diversification by generating new ecological opportunities through the evolution of novel predator-resistant strategies or in the form of access to predator-free space ( Nosil and Crespi, 2006 ; Meyer and Kassen, 2007 ). More importantly, bacterial strains were not equally susceptible to predation. They used different physical and chemical means against nematodes predation, such as bacterial cell shape, filamentation, biofilms as well as the production of pigments, polysaccharides and toxins ( Jousset et al., 2009 ; Jousset, 2011 ; Bjørnlund et al., 2012 ). In addition, bacterivores possessed selective feeding traits, which were largely determined by physical constraints of their feeding apparatus and specific detection of chemical cues produced by taxonomically different bacteria ( Bonkowski et al., 2009 ). Clearly, selective predation was fundamentally important for bacterivores to maximize their own fitness and elicit the influences on bacterial community dynamics.
Bulk soils are a resource-limited environment when compared with rhizosphere. Bacterivores–bacteria interactions were previously examined in bulk soils obtained from the same experimental sites ( Jiang et al., 2013 , 2014 ). The average total number of nematodes and total bacterial biomass in bulk soils were about half of those found in rhizosphere soils. Interestingly, nematodes and bacteria displayed the similar relationships in the rhizosphere and bulk soils, that is, positive correlations between bacterivores abundance and bacterial biomass as well as bacterial diversity. The data thus suggest that resource constraints may not be the major factor to determine the bacterivores–bacteria community interactions in soils.
In this work, our understanding on the ecological interactions between bacteria and bacterivorous nematodes has been extended to a specific functional group of ALP-producing bacteria. Our results revealed that bacterivores enhanced ALP-producing bacterial abundance and ALP activity, but produced no significant effects on ACP activity. This finding makes sense as soil ACPs were mostly derived from plant and fungi ( Tabatabai, 1994 ). Selective predation likely accounted for the significant effects of nematodes on determining ALP-producing bacterial community composition. For example, bacterivores abundance was positively correlated with Gammaproteobacteria ( r =0.878, P <0.001) and Betaproteobacteria ( r =−0.812, P <0.001), rather than Actinobacteria ( r =0.014, P =0.936). The data support the previous notion that bacterial-feeding nematodes prefer to feed on Gram-negative bacteria (for example, Pseudomonas , a typical rhizosphere colonizer) over Gram-positive bacteria, likely because their thinner cell walls are easier to be digested ( Salinas et al., 2007 ).
We examined the interaction networks between bacteria and bacterivorous nematodes, and identified Mesorhizobium as the keystone species for ALP activity across three aggregate fractions. These keystone species served as gatekeepers in the ecological functions of the bacterial community, with important contributions to biogeochemical cycling ( Lynch and Neufeld, 2015 ). It was different from the ammonia-oxidizing bacterial and archaeal community, which occupied two different keystone species (a module hub and a connector) in three aggregate fractions ( Montoya et al., 2006 ; Jiang et al., 2015 ). This result suggested that the ALP-producing bacterial community was more susceptible to nematode predation than the ammonia oxidizers. Manure applications promoted the formation of the LMA fraction, the intra-aggregate pore spaces of which were more suitable for bacterivorous nematode survival. Higher density of bacterivores population comprised the vast and complex networks of the nematodes–bacteria associations. The stronger positive effect of bacterivores on Mesorhizobium in the LMA probably grew more predominant contribution to ALP-producing bacterial abundance and ALP activity. Thus, the LMA network could be considered as a better-organized soil food wed with more functional interrelated bacterivorous nematodes and bacteria.
In conclusion, the data presented here showed that nematode predation promotes bacterial community dynamics in red soil, and the extent of the effects varied greatly at the level of soil aggregates. Specifically, the abundance of bacterivores was positively correlated with bacterial biomass and the levels of bacterial diversity. Moreover, nematode predation produced significant influences on species compositions of the bacterial community. In regards to the specific functional groups of ALP-producing bacteria, they displayed the similar effects as the total bacterial community. There was no sufficient evidence to suggest that ALP-producing bacteria were disproportionally affected (or specifically targeted) by nematodes. Interaction network analysis revealed significant effects of nematode on the keystone species of the bacterial community. More specifically, there was a positive correlation between the most dominant nematode Protorhabditis and the ALP-producing keystone 'species' Mesorhizobium . This may explain the findings that nematode grazing stimulated ALP activity. Finally, a systematic and comprehensive understanding of nematodes–bacteria interactions has been achieved at the level of soil aggregate. In general, microaggregates contain higher levels of bacterial abundance and diversity but less amount of bacterivorous nematodes compared with large macroaggregates. Conversely, nematode predation occurred more actively in large macroaggregates than in microaggregates. Together, nematode predation has an important role in determining the composition and dynamics of bacterial community in a spatially dependent manner.
Anderson MJ, Willis TJ . (2003). Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84 : 511–525.
Article Google Scholar
Anderson MJ . (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol 26 : 32–46.
Google Scholar
Barker KR . (1985). Nematode extraction and bioassays. In: Barker KR (ed). An Advanced Treatise on Meloidogyne Methodology , vol 2. North Carolina State University Graphics: Raleigh, NC, USA, pp 19–35.
Bjørnlund L, Liu MQ, Rønn R, Christensen S, Ekelund F . (2012). Nematodes and protozoa affect plants differently, depending on soil nutrient status. Eur J Soil Biol 50 : 28–31.
Bonkowski M . (2004). Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162 : 617–631.
Bonkowski M, Villenave C, Griffiths B . (2009). Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant soil 321 , 213–233.
Bongers T, Bongers M . (1998). Functional diversity of nematodes. Appl Soil Ecol 10 : 239–251.
Bronick CJ, Lal R . (2005). Soil structure and management: a review. Geoderma 124 : 3–22.
Article CAS Google Scholar
Brown DH, Ferris H, Fu S, Plant R . (2004). Modeling direct positive feedback between predators and prey. Theor Popul Biol 65 : 143–152.
Buchan D, Moeskops B, Ameloot N, de Neve S, Sleutel S . (2012). Selective sterilisation of undisturbed soil cores by gamma irradiation: effects on free-living nematodes, microbial community and nitrogen dynamics. Soil Biol Biochem 47 : 10–13.
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P . (2013). Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64 : 807–838.
Byrne BM . (2010) Structural Equation Modelling with AMOS: Basic concepts, applications, and programming , 2nd edn. Routledge: New York, NY, USA.
Chang A, Schomburg I, Placzek S, Jeske L, Ulbrich M, Xiao M et al . (2015). BRENDA in 2015: exciting developments in its 25th year of existence. Nucleic Acids Res 43 : 439–446.
Chesson P, Kuang JJ . (2008). The interaction between predation and competition. Nature 456 : 235–238.
Cressman R, Garay J . (2009). A predator-prey refuge system: evolutionary stability in ecological systems. Theor Popul Biol 76 : 248–257.
De'Ath G . (2007). Boosted trees for ecological modeling and prediction. Ecology 88 : 243–251.
Diehl S, Cooper SD, Kratz KW, Nisbet RM, Roll SK, Wiseman SW et al . (2000). Effects of multiple, predator–induced behaviors on short–term producer–grazer dynamics in open systems. Am Nat 156 : 293–313.
Djigal D, Brauman A, Diop T, Chotte JL, Villenave C . (2004). Influence of bacterial-feeding nematodes (Cephalobidae) on soil microbial communities during maize growth. Soil Biol Biochem 36 : 323–331.
Ettema CH, Wardle DA . (2002). Spatial soil ecology. Trends Ecol Evol 17 : 177–183.
Fraser TD, Lynch DH, Bent E, Entz MH, Dunfield KE . (2015a). Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol Biochem 88 : 137–147.
Fraser T, Lynch DH, Entz MH, Dunfield KE . (2015b). Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 257–258 : 115–122.
Frostegård A, Bååth E . (1996). The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22 : 59–65.
Fu S, Ferris H, Brown D, Plant R . (2005). Does the positive feedback effect of nematodes on the biomass and activity of their bacteria prey vary with nematode species and population size? Soil Biol Biochem 37 : 1979–1987.
Ingham RE, Trofymow J, Ingham ER, Coleman DC . (1985). Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55 : 119–140.
Jiang Y, Jin C, Sun B . (2014). Soil aggregate stratification of nematodes and ammonia oxidizers affects nitrification in an acid soil. Environ Microbiol 16 : 3083–3094.
Jiang Y, Sun B, Jin C, Wang F . (2013). Soil aggregate stratification of nematodes and microbial communities affects the metabolic quotient in an acid soil. Soil Biol Biochem 60 : 1–9.
Jiang Y, Sun B, Li H, Liu M, Chen L, Zhou S . (2015). Aggregate-related changes in network patterns of nematodes and ammonia oxidizers in an acidic soil. Soil Biol Biochem 88 : 101–109.
Jousset A . (2011). Ecological and evolutive implications of bacterial defences against predators. Environ Microbiol 14 : 1830–1843.
Jousset A, Rochat L, Péchy-Tarr M, Keel C, Scheu S, Bonkowski M . (2009). Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J 3 : 666–674.
Lynch MD, Neufeld JD . (2015). Ecology and exploration of the rare biosphere. Nat Rev Microbiol 13 : 217–229.
Meyer JR, Kassen R . (2007). The effects of competition and predation on diversification in a model adaptive radiation. Nature 446 : 432–435.
Montoya JM, Pimm SL, Solé RV . (2006). Ecological networks and their fragility. Nature 442 : 259–264.
Nannipieri P, Giagnoni L, Landi L, Renella G . (2011) Role of phosphatase enzymes in soil. In: Bunemann EK et al (ed). Phosphorus in Action Soil Biology 26 . Springer Verlag: Berlin/Heidelberg, Germany, pp 215–241.
Book Google Scholar
Neher DA . (2010). Ecology of plant and free-living nematodes in natural and agricultural soil. Annu Rev Phytopathol 48 : 371–394.
Newman MEJ . (2006). Modularity and community structure in networks. Proc Natl Acad Sci USA 103 : 8577–8582.
Nosil P, Crespi BJ . (2006). Experimental evidence that predation promotes divergence in adaptive radiation. Proc Natl Acad Sci USA 103 : 9090–9095.
Quénéhervé P, Chotte JL . (1996). Distribution of nematodes in vertisol aggregates under a permanent pasture in Martinique. Appl Soil Ecol 4 : 193–200.
Ranjard L, Richaume AS . (2001). Quantitative and qualitative microscale distribution of bacteria in soil. Res Microbiol 152 : 707–716.
Rillig MC, Mummey DL . (2006). Mycorrhizas and soil structure. New Phytol 171 : 41–53.
Rønn R, Vestergård M, Ekelund F . (2012). Interactions between bacteria, protozoa and nematodes in soil. Acta Protozool 51 : 223–235.
Sakurai M, Wasaki J, Tomizawa Y, Shinano T, Osaki M . (2008). Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci Plant Nutr 54 : 62–71.
Salinas KA, Edenborn SL, Sexstone AJ, Kotcon JB . (2007). Bacterial preferences of the bacterivorous soil nematode Cephalobus brevicauda (Cephalobidae): effect of bacterial type and size. Pedobiologia 51 : 55–64.
Six J, Elliott ET, Paustian K . (2000). Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32 : 2099–2103.
Spohn M, Kuzyakov Y . (2013). Distribution of microbial- and root derived phosphatase activities in the rhizosphere depending on P availability and C allocation–coupling soil zymography with 14C imaging. Soil Biol Biochem 67 : 106–113.
Tabatabai MA (1994). Soil enzymes. In: Weaver RW, Angle JS, Bottomley PS (eds). Methods of Soil Analysis, Part 2, Microbiological and Biochemical Properties . Soil Science Society of America: Madison, WI, USA, pp 775–833.
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A . (2013). Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fert Soils 49 : 661–672.
Tisdall J, Oades JM . (1982). Organic matter and water-stable aggregates in soils. J Soil Sci 33 : 141–163.
Trap J, Bonkowski M, Plassard C, Villenave C, Blanchart E . (2016). Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 398 : 1–24.
van der Heijden MG, Bardgett RD, van Straalen NM . (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11 : 296–310.
Yeates GW, Bongers T, de Goede RGM, Freckman DW, Georgieva SS . (1993). Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25 : 315–331.
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
We are very grateful to Wenqing Fan who helped cultivate nematode in microcosm experiment, and Haiyan Qian for her assistance in field experiments. This research was funded by National Key R&D Project (2016YDFD0200309), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15030201), National Basic Research Program of China (2014CB441003), Science and Technology Service Network Initiative (KFJ-SW-STS-142), and the Youth Innovation Promotion Association of CAS (2017361).
Author information
Authors and affiliations.
State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
Yuji Jiang, Jiabao Zhang, Yan Chen, Lijun Chen & Bo Sun
College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing, China
Manqiang Liu, Xiaoyun Chen & Huixin Li
Institute of Natural and Mathematical Sciences, Massey University at Albany, Auckland, New Zealand
- Xue-Xian Zhang
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Xue-Xian Zhang or Bo Sun .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Supplementary information (docx 28 kb), supplementary information (docx 3136 kb), rights and permissions.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
Reprints and permissions
About this article
Cite this article.
Jiang, Y., Liu, M., Zhang, J. et al. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J 11 , 2705–2717 (2017). https://doi.org/10.1038/ismej.2017.120
Download citation
Received : 26 February 2017
Revised : 21 May 2017
Accepted : 08 June 2017
Published : 25 July 2017
Issue Date : December 2017
DOI : https://doi.org/10.1038/ismej.2017.120
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impact of captivity and natural habitats on gut microbiome in epinephelus akaara across seasons.
- Fangyi Chen
- Ke-Jian Wang
BMC Microbiology (2024)
Conceptualizing soil fauna effects on labile and stabilized soil organic matter
- Gerrit Angst
- Anton Potapov
- Nico Eisenhauer
Nature Communications (2024)
Comparison of microbial communities in unleached and leached ionic rare earth mines
- Haitao Wang
- Chunqiao Xiao
Environmental Science and Pollution Research (2024)
Dynamic Changes in Rhizosphere Microbial Communities of Watermelon During Continuous Monocropping with Gravel Mulch
- Tingting Shen
Journal of Soil Science and Plant Nutrition (2024)
Introducing N2-fixing tree species into Eucalyptus plantations increases organic phosphorus transformation but decreases its accumulation within aggregates in subtropical China
- Haocheng Xu
- Xueman Huang
Plant and Soil (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Maintenance work is planned from 21:00 BST on Sunday 18th August 2024 to 21:00 BST on Monday 19th August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

RSC Sustainability
A comprehensive pre-treatment strategy evaluation of ligno-hemicellulosic biomass to enhance biogas potential in the anaerobic digestion process.

* Corresponding authors
a Gruner Renewable Energy Pvt Ltd, Noida, Uttar Pradesh, India E-mail: [email protected] , [email protected] , [email protected] , [email protected]
b Department of Biotechnology, Assam University, Silchar, India E-mail: [email protected]
Effective pretreatment of ligno-hemicellulosic biomass has emerged as a pre-requisite for its efficient conversion into biogas through the anaerobic digestion (AD) process. Assessment of various pre-treatment methods shows microbial pretreatment to be the most promising, economically viable, and environment-friendly option. Microbial pretreatment offers the advantages of low energy consumption and minimal pollution generation, thus making it a promising avenue for enhancing biogas yields from biomass. Fungi and bacteria, along with their enzymes, play pivotal roles in this method. Fungal pretreatment, involving cellulose and lignin-degrading species like brown-rot and white-rot fungi, have shown improved biogas yield. Bacterial and enzymatic pretreatments offer quicker results, making them attractive options for shortening the reaction time. Microbial consortia have shown remarkable efficiency in biomass degradation and its anaerobic digestion under thermophilic conditions. Physical pretreatment methods, such as mechanical size reduction, have shown potential to increase biomass accessibility and enhance biogas production. However, due to its energy-intensive nature and for improving biogas yields, further research is needed to develop more cost-effective approaches. The combination of physical and biological pretreatment methods offers a promising approach to effectively pretreat ligno-hemicellulosic biomass for improved biogas production.

- This article is part of the themed collection: RSC Sustainability Recent Review Articles
Transparent peer review
To support increased transparency, we offer authors the option to publish the peer review history alongside their article.
View this article’s peer review history
Article information
Download Citation
Permissions.
R. K. Prasad, A. Sharma, P. B. Mazumder and A. Dhussa, RSC Sustain. , 2024, Advance Article , DOI: 10.1039/D4SU00099D
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence . You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
share this!
August 8, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Microbes conquer the next extreme environment: Your microwave
by Frontiers

Since the industrial revolution, microbes have successfully colonized one novel type of habitat after another: for example, marine oil spills, plastic floating in the oceans, industrial brownfields, and even the interior of the International Space Station.
However, it turns out that one extreme environment harboring a specialized community of highly adapted microbes is much closer to home: inside microwaves. This finding has now been reported for the first time by researchers from Spain in a study in Frontiers in Microbiology . It's not only important from the perspective of hygiene, but could also inspire biotechnological applications—if the strains found inside microwaves can be put to good use in industrial processes that require especially hardy bacteria.
"Our results reveal that domestic microwaves have a more 'anthropized' microbiome, similar to kitchen surfaces, while laboratory microwaves harbor bacteria that are more resistant to radiation," said Daniel Torrent, one of the authors, and a researcher at the start-up Darwin Bioprospecting Excellence SL in Paterna, Spain.
Torrent and colleagues sampled microbes from inside 30 microwaves: 10 each from single-household kitchens, another 10 from shared domestic spaces--for example, corporate centers, scientific institutes, and cafeterias--and 10 from molecular biology and microbiology laboratories. The aim behind this sampling scheme was to see if these microbial communities are influenced by food interactions and user habits.
The team used two complementary methods to inventorize the microbial diversity : next-generation sequencing and cultivation of 101 strains in five different media.
A biodiverse microbiome right at home
In total, the researchers found 747 different genera within 25 bacterial phyla. The most frequently encountered phyla were Firmicutes, Actinobacteria, and especially Proteobacteria.
They found that the composition of the typical microbial community partly overlapped between shared domestic and single-household domestic microwaves, while laboratory microwaves were quite different. The diversity was lowest in single-household microwaves, and highest in laboratory ones.
Members of genera Acinetobacter, Bhargavaea, Brevibacterium, Brevundimonas, Dermacoccus, Klebsiella, Pantoea, Pseudoxanthomonas and Rhizobium were found only in domestic microwaves, whereas Arthrobacter, Enterobacter, Janibacter, Methylobacterium, Neobacillus, Nocardioides, Novosphingobium, Paenibacillus, Peribacillus, Planococcus, Rothia, Sporosarcina, and Terribacillus were found only in shared-domestic ones.
Nonomuraea bacteria were isolated exclusively from laboratory microwaves. There, Delftia, Micrococcus, Deinocococcus and one unidentified genus of the phylum Cyanobacteria were also common, found in significantly greater frequencies than in domestic ones.
The authors also compared the observed diversity with that in specialized habitats reported in the literature. As expected, the microbiome in microwaves resembled that found on typical kitchen surfaces.
"Some species of genera found in domestic microwaves, such as Klebsiella, Enterococcus and Aeromonas, may pose a risk to human health. However, it is important to note that the microbial population found in microwaves does not present a unique or increased risk compared to other common kitchen surfaces," said Torrent.
Parallel evolution
However, it was also similar to the microbiome in an industrial habitat: namely, on solar panels. The authors proposed that the constant thermal shock, electromagnetic radiation , and desiccation in such highly irradiated environments has repeatedly selected for highly resistant microbes, in the same manner as in microwaves.
"For both the general public and laboratory personnel, we recommend regularly disinfecting microwaves with a diluted bleach solution or a commercially available disinfectant spray. In addition, it is important to wipe down the interior surfaces with a damp cloth after each use to remove any residue and to clean up spills immediately to prevent the growth of bacteria," recommended Torrent.
Journal information: Frontiers in Microbiology
Provided by Frontiers
Explore further
Feedback to editors
Zebrafish use surprising strategy to regrow spinal cord: Findings could help identify ways to heal spinal cord damage
6 minutes ago

Novel light transport model improves X-ray phase contrast imaging
12 hours ago
NASA telescopes work out black hole's feeding schedule

Geochemistry study links ancient anorthosites to early Earth's hot subduction
13 hours ago

Tropical Atlantic mixing rewrites climate pattern rules
Protons can tune synaptic signaling by changing the shape of a protein receptor
14 hours ago

Scientists create material that can take the temperature of nanoscale objects

Findings challenge current understanding of nitrogenases and highlight their potential for sustainable bioproduction
15 hours ago

NASA still deciding whether to keep 2 astronauts at space station until next year

Statistical analysis can detect when ChatGPT is used to cheat on multiple-choice chemistry exams
Relevant physicsforums posts, therapeutic interfering particle.
16 hours ago
Cannot find a comfortable side-sleeping position
19 hours ago
Neutron contamination threshold in tissue using LINAC
Aug 8, 2024
Contradictory statements made by two different professors about IQ scores
Aug 2, 2024
New and Interesting Publications Relevant to the Origin of Life
The cass report (uk).
Jul 30, 2024
More from Biology and Medical
Related Stories

Bacteria in kitchen may not be as harmful as you think
Jul 10, 2023

Microwaves heat the soil to eliminate pests and help farmers manage soil diseases
Dec 19, 2023

Exposure to high-powered microwave frequencies may cause brain injuries
Apr 25, 2022
Microwaves used to deactivate coronavirus, flu, other aerosolized viruses
Jan 26, 2021

Solar minimum surprisingly constant
Nov 17, 2017

Microwave-powered rocket propulsion investigated
Jul 26, 2021
Recommended for you
Newly discovered ability of comammox bacteria could help reduce nitrous oxide emissions in agriculture

Earth's oldest, tiniest creatures are poised to be climate change winners—and the repercussions could be huge
17 hours ago

Revealing the mysteries within microbial genomes with a new high-throughput approach
An affordable tracking microscope to democratize microorganism research

City birds found to be carriers of antimicrobial resistant bacteria

Advance in stem cell therapy: New technique for manipulating stem cells opens door to novel treatments
Aug 13, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
COMMENTS
The high microbial biomass values found in the Biotree-FD experiment could be linked to higher clay content and lower potential evapotranspiration. The second axis explained 36.2% of the variance and was mainly affected by the seasonality of temperature, soil pH and tree density, which are variables that strongly increased the carbon-use ...
Microbial biomass carbon (MBC, µg carbon g −1 DM) measured by chloroform fumigation, was neither significantly affected by single or combined warming and elevated CO 2 in any season ...
We first show that the relationship between soil microbial biomass and diversity follows a unimodal (humped-back) pattern across global biomes (Fig. 1, Table S2).Moreover, the humped-back ...
The experiment was created in 2002 on a former arable field that had been under continuous cropland management for more than 40 years. ... Microbial biomass carbon concentrations were determined as the difference between fumigated and unfumigated soil samples measured by the TOC/TN analyzer by using an extraction factor k EC of 0.45 ...
a-c, Mean (±s.e., n = 3) modelled responses of soil carbon (a), microbial respiration (b) and microbial biomass C (c) to 50 years of simulated warming or a control scenario.d, Relationship ...
Microbial biomass carbon concentrations were determined as the difference between fumigated and unfumigated soil samples measured by the TOC/TN analyzer by using an extraction factor k ... Long‐term study of root biomass in a biodiversity experiment reveals shifts in diversity effects over time. Oikos, 123 (12), 1528-1536. 10.1111/oik ...
ration and soil microbial biomass datafrom a series of short and long-term laboratory incubation experiments with 13C labeled substrates to examine how plant communities (forest vs. grassland), edaphic properties, and microbial communities influence C cycling and t. e long-term fate of C in soil systems. Paired forest and grassland soils from ...
Changes in precipitation represent a major effect of climate change on tropical forests, which contain some of the earth's largest terrestrial carbon (C) stocks. Such changes are expected to influence microbes, nutrients, and the fate of C in tropical forest soils. To explore this, we assessed soil microbial biomass, potential extracellular enzyme activities, and nutrient availability in a ...
experiment. 13C incorporation into microbial biomass increased with land use intensification in low pH soils but decreased in high pH soils, impacting ecosystem carbon use efficiency (CUE) in opposing directions. Reduction in biosynthesis traits across land use intensity contrasts was due to increased abundance of proteins linked
Here we report on two independent laboratory experiments to explore short-term temperature and soil moisture effects on soil microbial physiology (i.e. respiration, growth, CUE, and microbial biomass turnover): (i) a temperature experiment with 1-day pre-incubation at 5, 15 and 25 °C at 60% water holding capacity (WHC), and (ii) a soil ...
The effects of soil type and organic material quality on the microbial biomass and functional diversity of cropland soils were studied in a transplant experiment in the same climate during a 1-year field experiment. Six organic materials (WS: wheat straw, CS: corn straw, WR: wheat root, CR: corn root, PM: pig manure, CM: cattle manure), and three contrasting soils (Ferralic Cambisol, Calcaric ...
Soil microbial biomass and community composition as affected by cover crop diversity in a short-term field experiment on a podzolized Stagnosol-Cambisol ... an on-farm experiment was conducted at a podzolized Stagnosol-Cambisol during seven months growing oil radish as single cover crop and five different cover crop mixtures comprising 5 to 13 ...
The fertilization experiment studied here is conducted in a grassland at the agricultural research station in Gumpenstein, Austria (49°29′37″N, 14°06′10″E). ... and were measured using a TOC analyzer (TOC-VCPH/CPNTNM-1, Shimadzu). Microbial biomass C and N was calculated with a conversion factor of 2.22 (Wu et al., 1990).
Purpose Cultivation can affect soil microbial activities, with consequences for microorganisms that metabolize soil organic carbon and release CO2 to the atmosphere. Nevertheless, there is limited understanding of the short-term effects that tillage disturbance, maize planting, or their interactions exert on microbial biomass and metabolic function in a typical karst calcareous soil. A 1-year ...
By conducting cross-validation experiments (a 16-year N and a 6-year acid addition) in a temperate semi-arid grassland, we tested the responses of soil microbial attributes (e.g., diversity and relative abundance) at various levels (community, phylum/class, and phylotype) to N and acid addition.
Half of the microbial biomass is in the uppermost (15 cm) soil, which stores <4% organic matter, and plays a major role in the breakdown and soil carbon pool building.
Microbial biomass C and N, EOC, EN, ... In the same experiment using a larger selection of sites (73, including the 10 sites in this study), plant richness increased on average by 8.3 species 5 yr after sward disturbance combined with sowing (Freitag et al., 2021). The increase in species richness was negatively related to plant aboveground ...
Microbial biomass, organic matter mineralization and nitrogen in soils from long-term experimental grassland plots (Palace Leas meadow hay plots, UK) ... different annual manure and fertilizer applications since 1897 and represent the second oldest permanent grassland experiment in the world. Although the soil properties have diverged as a ...
The present study aimed to contribute to the insufficient knowledge of functional and structural soil microbial properties influenced by organic and inorganic fertilisation and climatic conditions at two European long-term field experiments. Soil microbial biomass, activities of alkaline phosphatases, β-glucosidases and proteases, and ...
Methods for measuring soil microbial biomass using substrate-induced respiration and fumigation-extraction were the first ones to be standardised in the field of soil ... There are several precedents such as the minimum information about a proteomics experiment (Taylor et al., 2007) or the minimum information about a microarray ...
Many microbiological experiments are performed under conditions designed to maximize experimental simplicity or biomass yield (for example, at the end of exponential growth in complex media).
Microbial C uptake and allocation (13 C tracing)To trace 13 C into extractable organic C, MBC, CO 2, and PLFAs, the sets designated for 13 C-tracing were amended either with 10 atm% 13 C-labelled glucose solution or natural abundance water at timepoint zero and 144 h of the cooling experiment. To add the solutions and start the 13 C assay, the samples were briefly removed from the incubators ...
microbial biomass can be described using the first-order Michaelis-Menten equation. k = (2.303/t)log(x/x-a) where k is the turnover factor, t is time, x is the dry weight of the microbial biomass pool size, and a is the production of new material per hour. The turnover time of the microbial biomass.
Soil microbial biomass carbon and nitrogen (SMBC, SMBN) in Ferralic Cambisol, Calcaric Cambisol, and Luvic Phaeozem at the end of 1st and 12th months with the amendment of different organic materials. ... As the experiment proceeded, the amount of available C and N sources decreased and further entered the environment, e.g., as C and N gaseous ...
Global fertilizer production and mismanagement significantly contribute to many harmful environmental impacts, revealing the need for a greater understanding of crop growth and nutrient uptake, which can be used to optimize fertilizer management. This study experimentally adapts first-principles microbial modeling techniques to the hydroponic cultivation of Bibb lettuce (Lactuca sativa) under ...
Microbial biomass. Microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), dissolved organic carbon (DOC), and dissolved organic nitrogen (DON) concentrations were measured using the direct chloroform fumigation extraction method modified from Vance et al. and described in Hargreaves and Hofmockel .
For example, nematodes can stimulate microbial activity, resulting in either an increase or a decrease of microbial biomass in microcosm experiments (Trap et al., 2016).
Assessment of various pre-treatment methods shows microbial pretreatment to be the most promising, economically viable, and environment-friendly option. Microbial pretreatment offers the advantages of low energy consumption and minimal pollution generation, thus making it a promising avenue for enhancing biogas yields from biomass.
After 72 h of the inhibitor experiment, the bacterial biomass was centrifuged and harvested, then washed three times with sterile water, dried at −70 °C, and analyzed for the content of carbon in the bacterial biomass. ... Microbial growth, metabolism, and ecological functions could be affected by C/N ratios in the growth process of ...
The team used two complementary methods to inventorize the microbial diversity: ... ALICE measures interference pattern akin to the double-slit experiment. 10 minutes ago.