An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Dopamine Hypothesis of Drug Addiction and Its Potential Therapeutic Value
Marco diana.
- Author information
- Article notes
- Copyright and License information
Edited by: Lorenzo Leggio, Brown University, USA
Reviewed by: Diana Martinez, Columbia University, USA; Frederic Woodward Hopf, University of California at San Francisco, USA
*Correspondence: Marco Diana, ‘G. Minardi’ Cognitive Neuroscience Laboratory, Department of Drug Sciences, University of Sassari, Via Muroni n. 23, Sassari, Italy. e-mail: [email protected]
This article was submitted to Frontiers in Addictive Disorders, a specialty of Frontiers in Psychiatry.
Received 2011 Sep 14; Accepted 2011 Nov 2; Prepublished 2011 Oct 7; Collection date 2011.
This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially exploitable therapeutically. In particular, basic studies have documented a reduction in the electrophysiological activity of DA neurons in alcohol, opiate, cannabinoid, and other drug-dependent rats. Further, DA release in the Nucleus accumbens (Nacc) is decreased in virtually all drug-dependent rodents. In parallel, these studies are supported by increments in intracranial self stimulation (ICSS) thresholds during withdrawal from alcohol, nicotine, opiates, and other drugs of abuse, thereby suggesting a hypofunction of the neural substrate of ICSS. Accordingly, morphological evaluations fed into realistic computational analysis of the medium spiny neuron of the Nacc, post-synaptic counterpart of DA terminals, show profound changes in structure and function of the entire mesolimbic system. In line with these findings, human imaging studies have shown a reduction of dopamine receptors accompanied by a lesser release of endogenous DA in the ventral striatum of cocaine, heroin, and alcohol-dependent subjects, thereby offering visual proof of the “ dopamine-impoverished” addicted human brain. The lasting reduction in physiological activity of the DA system leads to the idea that an increment in its activity, to restore pre-drug levels, may yield significant clinical improvements (reduction of craving, relapse, and drug-seeking/taking). In theory, it may be achieved pharmacologically and/or with novel interventions such as transcranial magnetic stimulation (TMS). Its anatomo-physiological rationale as a possible therapeutic aid in alcoholics and other addicts will be described and proposed as a theoretical framework to be subjected to experimental testing in human addicts.
Keywords: addiction, dopamine, rTMS, dopamine agents, VTA, prefrontal cortex
Drug addiction is a brain disease that produces profound modifications in human behavior (Hyman, 2007 ; Koob and Volkow, 2010 ), with important negative consequences at various levels, including personal health, employment, family interactions, and society in general (Chandler et al., 2009 ). Therapeutic possibilities for this devastating illness are, with some rare exceptions, limited to pharmacologic treatments that are largely unsatisfactory (Koob et al., 2009 ; Leggio et al., 2010 ; Swift, 2010 ). From here the necessity to develop new therapeutic hypothesis/interventions independent from those commonly employed.
Transcranial magnetic stimulation (TMS), through generation of an electromagnetic field capable of crossing painlessly through the skull and influencing the underlying brain matter, appears to be a promising candidate for treating addictive behaviors (Barr et al., 2008 ; Feil and Zangen, 2010 ) and other brain diseases (Kobayashi and Pascual-Leone, 2003 ). In brief, this relatively new method allows modulation of discrete brain areas of the awake and conscious subject under study. The pulsatile electromagnetic field generated around the coil crosses the skull and is capable of directly exciting/inhibiting neurons in the underlying cortices (Padberg and George, 2009 ). Commonly employed as a research tool, TMS is recently affirming its role as a potential therapeutic means approved by the Food and Drug Administration for brain pathologies such as drug-resistant major depression, bipolar syndrome, and negative symptoms of schizophrenia. In the drug addiction field, the therapeutic potential of TMS has been tested in nicotine-dependent subjects (Lang et al., 2008 ; Amiaz et al., 2009 ), cocaine addicts (Boutros et al., 2001 , 2005 ; Sundaresan et al., 2007 ; Politi et al., 2008 ), and alcoholics (Conte et al., 2008 ; Mishra et al., 2010 ). Although the results are certainly encouraging, the disparity of clinical outcomes evaluated in different studies and diversity of pattern/site/methodology of stimulation precludes direct comparisons and hampers firm conclusions. However, in those studies in which craving was measured (Politi et al., 2008 ; Amiaz et al., 2009 ; Mishra et al., 2010 ) significant reductions have been found, thus encouraging further experimental scrutiny. At present, we are evaluating anti-craving and alcohol-intake efficacy of TMS in alcoholics (Addolorato et al., in preparation), short and long-term cocaine intake in treatment-seeking cocaine addicts (Pedetti et al., in preparation), and money/cocaine choice in a lab study of cocaine addicts non-seeking treatment (Martinez et al., in preparation). Nevertheless, the brain site(s) to be stimulated/inhibited and the stimulation parameters (i.e., frequency of stimulation, number of session etc.,) are matters of intense debate and an appropriate rationale is needed.
Dopamine as a Possible Therapeutic Target
The role of central DA systems in the acute effects of drugs of abuse was recognized long ago (Wise, 1980 , 1987 ; Di Chiara and Imperato, 1988 ). Even before (Ahlenius et al., 1973 ), attempts were made to prevent human alcohol-induced euphoria through administration of the DA synthesis inhibitor alpha methyl-para-tyrosine. Although theoretically ineccepibile, this approach (reduction of drug-induced DA increments to prevent abuse) is unlikely to have a practical validity as any compound with DA antagonistic (i.e., neuroleptics) properties is known to be aversive in humans. On the other hand, widely documented experimental evidence suggests that the mesolimbic dopamine system is “hypofunctional” in the addicted brain (Melis et al., 2005 ). In brief, the hypothesis contends that decreased DA function in addicted subjects results in a decreased interest to non-drug-related stimuli and increased sensitivity to the drug of choice (Melis et al., 2005 ), leading to propose that restoring DA function might be therapeutically advantageous.
Alcohol-dependent (in the present context the term “dependent,” when referred to a non-human experimental subject, indicates a condition in which the subject has shown unequivocally a proof of dependency, i.e., somatic signs of withdrawal) rats show a profound reduction of spontaneous firing rate and burst firing of antidromically identified Nucleus accumbens (Nacc)-projecting ventral tegmental area (VTA) DA-containing neurons in rats (Diana et al., 1993 ) and mice (Bailey et al., 2001 ) resulting in a concomitant reduction of microdialysate DA in the Nacc (Rossetti et al., 1992 ; Diana et al., 1993 ; Barak et al., 2011 ). Further, the reduced dopaminergic activity outlasts somatic signs of alcohol-withdrawal (Diana et al., 1996 , 2003 ) thereby suggesting a role for DA in the lasting consequences of alcohol dependence while excluding the possibility of a DA role in somatic aspects of withdrawal. Further, original (pre-dependence) DA levels in the Nacc are restored when ethanol is self (Weiss et al., 1996 ) and/or passively administered (Diana et al., 1993 , 1996 ). These observations are paralleled by intracranial self stimulation (ICSS) studies showing that ethanol-withdrawn rats are capable of maintaining the ICSS behavior provided that the stimulus current intensity is increased (Schulteis et al., 1995 ). This important observation strongly indicates that the neural substrate responsible for maintaining the ICSS behavior is hyperpolarized, or more refractory, in the alcohol-dependent subject as compared with its control. Since the neural substrate of ICSS involves DA axons (Yeomans, 1989 ; Yeomans et al., 1993 ) near the stimulating electrode, the results are complementary to those reported above and well support a deficitary function of DA neurons. In addition, the perseverance of the reduction in DA activity (beyond resolution of somatic signs of withdrawal) has also been documented in morphine-dependent rats (Diana et al., 1999 ), while a dichotomy between DA function and somatic withdrawal has been observed in cannabinoid–withdrawn rats (Diana et al., 1998 ). Similarly, conditioned heroin withdrawal decreases reward sensitivity (Kenny et al., 2006 ) which persists well beyond the initial phase of withdrawal. These findings, observed across different addicting compounds and experimental conditions, suggest that DA hypofunction persists over time, although reverting to “normality” (Diana et al., 1999 , 2006 ), eventually with species-specific time course.
In addition to basic literature, reports in humans are also supportive of a compromised role of DA transmission in alcoholics. While alcohol increases DA release in healthy subjects (Boileau et al., 2003 ) with some gender differences (Urban et al., 2010 ), a reduced number of DA receptors has been observed (Volkow et al., 1996 ; Martinez et al., 2005 ) in alcoholics that appears to be accompanied by a blunted DA release (Martinez et al., 2005 , 2007 ; Volkow et al., 2007 ). While the reduced number of DA receptors could be, at first sight, be viewed as suggesting an increased DA release, it should be noted that by administering the DA inhibitor alpha methyl-para-tyrosine, Martinez et al. ( 2009 ) were able to exclude this possibility. Indeed, while healthy controls do show an increased raclopride binding after acute alpha methyl-para-tyrosine administration, cocaine-dependent subjects do not (or to a significantly lesser extent; Martinez et al., 2009 ). Similar results were obtained with the dopamine releasing agent methylphenidate (Volkow et al., 2007 ) and amphetamine (Martinez et al., 2005 ) in alcoholics. Notably, artificially increasing the brain levels of DAD2 receptors, using a replication-deficient adenoviral vector containing the rat cDNA insert for DAD2 into the Nacc, reduces alcohol intake in spontaneously drinking rats, thereby offering the counterproof that a potentiation of DA transmission may have beneficial effects on alcohol-seeking and alcohol-taking, in experimental models (Thanos et al., 2001 , 2004 ). In line with this conclusion, a spontaneous high number of DA D2 receptors has been shown to have a protective role in non-alcoholic members of alcoholic families (Volkow et al., 2006 ). These findings further support the notion that the number of DA receptors (and consequently DA transmission) inversely correlates with alcohol drinking.
These observations may suggest that “ boosting” DA neurons to produce more available DA in the synaptic cleft could alleviate some of the symptoms of addiction and alcoholism, thereby acquiring a therapeutic character. In theory, this could be achieved by two different strategies: (1) DA-potentiating drugs and (2) TMS. Both possibilities are discussed below.
Dopamine-Potentiating Drugs
Although medications that increase DA activity could be effective in treating alcohol abuse disorders, conflicting results have been produced (Swift, 2010 ). For example, it was suggested that the DA agonist bromocriptine reduced drinking in alcoholics (Lawford et al., 1995 ), but a randomized, double-blind, placebo-controlled study using a long-acting injectable bromocriptine preparation in 366 alcoholic-dependent individuals did not find difference in alcohol relapse between medication and placebo (Naranjo et al., 1997 ). Another example is the stimulant medication modafinil (DA indirect agonist), found to improve cognition in 40 alcoholics with organic brain syndrome, but effects on drinking could not be measured (Saletu et al., 1990 ). However, modafinil reduced cocaine use in a placebo-controlled study with 62 cocaine-dependent individuals (Dackis and O’Brien, 2005 ), while another trial did not find differences between modafinil and placebo tested for methamphetamine users (Shearer et al., 2010 ). While evidence for the use of DA agonists as a treatment for alcohol and/or substance use disorders is inconclusive (Swift, 2010 ), there has been a revived interest for these drugs, possibly because adequate neurobiological rationale (Melis et al., 2005 ) is now available. For example, aripiprazole (Semba et al., 1995 ; Burris et al., 2002 ; Shapiro et al., 2003 ) a partial DA agonist which in principle should antagonize DA when tone is high, whereas should increase DA transmission when basic tone is low, represents a proposed treatment for alcohol abuse disorders (Kenna et al., 2009 ). Human laboratory alcohol studies have shown that aripiprazole reduces drinking (Kranzler et al., 2008 ), especially in the more impulsive alcoholic (Voronin et al., 2008 ). An fMRI study demonstrated that aripiprazole significantly attenuates neural activity in the ventral striatum in response to alcohol cues (Myrick et al., 2010 ) thereby suggesting a therapeutic potential for cue-induced relapse. Further, a 12-week, double-blind, placebo-controlled treatment study with 295 alcohol-dependent individuals found that aripiprazole initially decreased heavy drinking days compared to placebo, but this significant effect was not present when the target dose of 30 mg was reached (Anton et al., 2008 ). This trial also showed greater side-effects and greater study discontinuation in the aripiprazole arm, as compared to placebo (Anton et al., 2008 ). Interestingly, an open-label study of aripiprazole (Martinotti et al., 2009 ) and a recent human laboratory study (Kenna et al., 2009 ) suggests that lower doses of aripiprazole (5–15 mg per day) may be better tolerated and still reduce drinking with effects on relapse comparable to those obtained with the opiate antagonist naltrexone (Martinotti et al., 2009 ).
In summary, dopamine plays a key role in the addiction process, but significant side-effects have limited the use of medications that work directly on the dopaminergic system. The use of DA partial agonists with lower side effect profiles, and appropriate dosing represent important directions for future research in this area.
Transcranial Magnetic Stimulation
Increasing DA tone with appropriate pharmacological tools, is only one of the possible strategies. Endogenous activity of DA-containing neurons can be augmented with non-pharmacological tools such as TMS (Strafella et al., 2001 ) thereby providing, in principle, an adjunct to the “therapeutic arsenal” against addiction, endowed with lesser systemic side-effects and limited contraindications. However, while the rationale is “neurochemical” for pharmacological agents (neurotransmitter receptors, brain area etc.,) , it must be anatomically based for TMS. Being that DA-containing neurons are located deeply in the brainstem (thereby making the neurons inaccessible to direct TMS stimuli) it becomes unavoidable to reach them indirectly through neurons located elsewhere in the brain. The dorsolateral prefrontal cortex (DLPfcx) by projecting monosynaptically to the rat (Carr and Sesack, 2000 ) and primate (Frankle et al., 2006 ) VTA may serve this function. These studies show a projection from the PFC to midbrain DA neurons, terminating both within the SN proper as well as in the VTA. They arise from a broad region of the PFC, including the DLPfcx, cingulate, and orbital cortices. Indeed, these pyramidal neurons (Figure 1 ) could be exploited as the primary target of the TMS stimulus and their increased activity to produce, ultimately, an enhancement in DA availability in the synaptic cleft in the Nacc. Schematically, the hypothesized circuit (Figure 2 ) would be the following: TMS → DLPfcx → VTA → DA increase in forebrain projection site (i.e., Nacc). In this context, it is imperative to employ stimulation parameters consonant with the physiological activity of the system under study to restore pre-drug DA levels. For instance, it has been shown that DLPfcx stimulation produces bursts in rat DA neurons (Gariano and Groves, 1988 ; Murase et al., 1993 ), highlighting the importance of stimulation parameters. Indeed, burst firing is more efficacious than single spiking (of identical frequency but evenly spaced action potentials) in inducing DA release in terminal areas (Gonon, 1988 ; Manley et al., 1992 ). Consistently, the role of DLPfcx in regulating basal DA activity through the VTA has been reported (Taber et al., 1995 ; Karreman and Moghaddam, 1996 ).
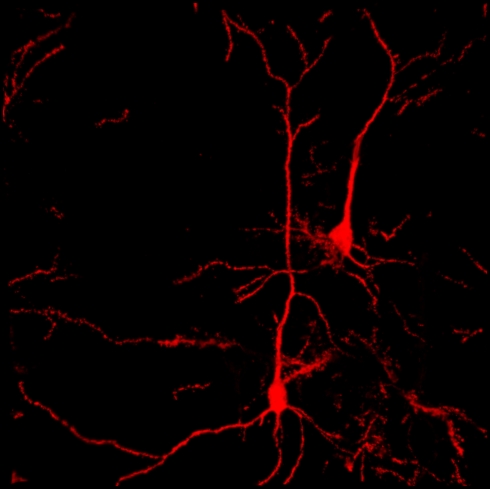
Confocal reconstruction of Golgi-stained pyramidal neurons from DLPfcx obtained by a projection of 55 scans for a depth of 27.5 μm in the z -axis . DLPfxc may represent a useful target for rTMS stimulation.
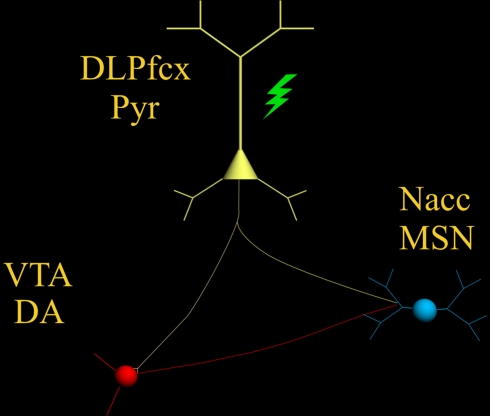
The scheme illustrates the proposed circuit to be activated by the TMS stimulus (green) which, by activating the pyramidal neuron (yellow) with its neurotransmitter glutamate, would excite: (1) DA-containing neurons of the VTA (red) and (2) MSN of the Nacc (blue) .
Among the various factors that are likely to influence its efficacy, the importance of the baseline cortical activation state on the impact of TMS is fundamental (Silvanto and Pascual-Leone, 2008 ). This state-dependency is key as the neural impact of any external stimulus represents an interaction with the ongoing brain activity at the time of stimulation. The effects of any external stimulus are therefore not only determined by the properties of that stimulus but also by the activation state of the brain. Accordingly, it has been shown that baseline cortical activity determines whether TMS hampers or hastens behavior (Silvanto et al., 2008 ). The state-dependency principle described above would also apply to the state of the DA system. The hypodopaminergic state (Melis et al., 2005 ) should then “amplify” the effect of TMS as compared with that expected in a normo-functioning DA system.
The responsivity of the neuron(s) to electrical and synaptic stimuli is strictly dependent on its morphological features, which in turn, are deeply modified by drugs of abuse (Robinson and Kolb, 2004 ) and withdrawal from chronic treatment with opiates (Sklair-Tavron et al., 1996 ; Spiga et al., 2003 , 2005 ), cannabis derivatives/analogs (Spiga et al., 2010 ), and psychostimulants (Robinson and Kolb, 1997 ) have been shown to produce reductions in DA cells size (Sklair-Tavron et al., 1996 ; Spiga et al., 2003 ), paralleled by persistently (Diana et al., 2006 ) altered patterns of synaptic connectivity, and spines density in the Nacc and Pfcx (Robinson and Kolb, 1997 ). These architectural changes would be expected to modify intrinsic spontaneous action potential generating capacity and responsiveness of the system to the TMS stimuli. Accordingly, realistic computational analysis (Spiga et al., 2010 ) of cannabis-dependent rats, generated by input of experimentally verified morphometrical and electrophysiological properties, predicts a lower action potential generation of Nacc medium spiny neuron (MSN). These results suggest that MSN, of cannabis-dependent rats are likewise hypofunctional. Considering that the main drive of these neurons is cortical glutamate (Glu; see discussion in Spiga et al., 2010 , and references therein; Kalivas and Hu, 2006 ) it raises the possibility of a reduction of Glu as a causal factor. This finding, thus offers the additional possibility that stimulation of these units through TMS may be advantageous in restoring pre-drug physiological activity. Indeed, TMS cortical application should increase the activity of glutamate-containing cortico-fugal fibers monosynaptically impinging upon the spine’s heads of Nacc MSN (Groenewegen et al., 1991 ). Considering the fundamental role Glu plays in synaptic plasticity (Russo et al., 2010 ), its role could also be exploited in LTP-like stimulation parameters, ultimately aimed at producing lasting and enduring restoration of original physiological activity. These characteristics must be considered and coherently inserted into a framework to obtain optimal stimulation parameters. In vivo recordings of VTA-projecting DLPfcx neurons do fire spontaneously around 4–6 Hz (Pistis et al., 2001 ) and a TMS stimulus frequency of 10 Hz could be a reasonable frequency to obtain a significant increase in VTA-projecting neurons aimed at stimulating the “ deficient” dopamine system and its post-synaptic counterpart (i.e., MSN of the Nacc).
Another factor to be considered is that all previous studies (see above) applied the TMS stimulus monolaterally, yet obtaining a reduction of alcohol craving (Mishra et al., 2010 ). While alcohol intake was not measured, and contralateral effects cannot be excluded a priori , it is possible that application of TMS bilaterally, as in the case of the H-coil (Feil and Zangen, 2010 ), would yield stronger cortical activation (larger number of fibers activated) with an increased probability of a significant increment of bilateral DA release. It should be noted that unilateral TMS application has already been reported to increase DA release (Strafella et al., 2001 ) omolaterally in the human striatum, as well as in rodents (Keck et al., 2002 ; Zangen and Hyodo, 2002 ), and even in morphine-withdrawn rats (Erhardt et al., 2004 ), thereby supporting the rationale outlined above. Although Strafella et al. ( 2001 ) proposed activation of (Glu-containing) cortico-fugal fibers making synaptic contact with DA-containing terminals in the ventral striatum, to explain their results, it should be noted that the existence of axo-axonic contacts has always being questioned based on the lack of appropriate anatomical observations (Groenewegen et al., 1991 ; Meredith et al., 2008 ).
While many technical details for optimal stimulation parameters need further investigation and optimization, the TMS appears to deserve careful experimental scrutiny as a potential therapeutic tool in alcoholics and other addicts. Indeed, with its nearly absent systemic effects, minimal side-effects, and a low degree of invasiveness, TMS may offer the first opportunity for an efficacious, non-pharmacological, therapeutic tool in alcoholism and other chemical dependencies. If appropriately combined with a solid neurobiological rationale (DA system), it may offer a unique opportunity for developing further the first “ electrophysiological” approach in studying and eventually treating the devastating and widespread brain disease of addiction.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported, in part, by grants from MIUR (PRIN. N°2004052392) and Dipartimento Politiche Antidroga. The author wish to thank S. Spiga for elaborating iconographic material presented.
- Ahlenius S., Carlsson A., Engel J., Svensson T., Södersten P. (1973). Antagonism by alpha methyltyrosine of the ethanol-induced stimulation and euphoria in man. Clin. Pharmacol. Ther. 14, 586–591 [ DOI ] [ PubMed ] [ Google Scholar ]
- Amiaz R., Levy D., Vainiger D., Grunhaus L., Zangen A. (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104, 653–660 10.1111/j.1360-0443.2008.02448.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Anton R. F., Kranzler H., Breder C., Marcus R. N., Carson W. H., Han J. (2008). A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J. Clin. Psychopharmacol. 28, 5–12 10.1097/jcp.0b013e3181602fd4 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bailey C. P., O’Callaghan M. J., Croft A. P., Manley S. J., Little H. J. (2001). Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology 41, 989–999 10.1016/S0028-3908(01)00146-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- Barak S., Carnicella S., Yowell Q. W., Ron D. (2011). Glial cell line-derived neurotrophic factor reverses alcoho-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J. Neurosci. 31, 9885–9894 10.1523/JNEUROSCI.1750-11.2011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barr M. S., Fitzgerald P. B., Farzan F., George T. P., Daskalakis Z. J. (2008). Transcranial magnetic stimulation to understand the pathophysiology and treatment of substance use disorders. Curr. Drug Abuse Rev. 1, 328–339 10.2174/1874473710801030328 [ DOI ] [ PubMed ] [ Google Scholar ]
- Boileau I., Assaad J. M., Pihl R. O., Benkelfat C., Leyton M., Diksic M., Tremblay R. E., Dagher A. (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 15, 226–231 10.1002/syn.10226 [ DOI ] [ PubMed ] [ Google Scholar ]
- Boutros N. N., Lisanby S. H., McClain-Furmanski D., Oliwa G., Gooding D., Kosten T. R. (2005). Cortical excitability in cocaine-dependent patients: a replication and extension of TMS findings. J. Psychiatr. Res. 39, 295–302 10.1016/j.jpsychires.2004.07.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- Boutros N. N., Lisanby S. H., Tokuno H., Torello M. W., Campbell D., Berman R., Malison R., Krystal J. H., Kosten T. (2001). Elevated motor threshold in drug-free, cocaine-dependent patients assessed with transcranial magnetic stimulation. Biol. Psychiatry 49, 369–373 10.1016/S0006-3223(00)00948-3 [ DOI ] [ PubMed ] [ Google Scholar ]
- Burris K. D., Molski T. F., Xu C., Ryan E., Tottori K., Kikuchi T., Yocca F. D., Molinoff P. B. (2002). Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 302, 381–389 10.1124/jpet.102.033175 [ DOI ] [ PubMed ] [ Google Scholar ]
- Carr D. B., Sesack S. R. (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 20, 3864–3873 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chandler R. K., Fletcher B. W., Volkow N. D. (2009). Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA 301, 183–190 10.1001/jama.2008.976 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Conte A., Attilia M. L., Gilio F., Iacovelli E., Frasca V., Bettolo C. M., Gabriele M., Giacomelli E., Prencipe M., Berardelli A., Ceccanti M., Inghilleri M. (2008). Acute and chronic effects of ethanol on cortical excitability. Clin. Neurophysiol. 119, 667–674 10.1016/j.clinph.2007.10.021 [ DOI ] [ PubMed ] [ Google Scholar ]
- Dackis C., O’Brien C. (2005). Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 8, 1431–1436 10.1038/nn1105-1431 [ DOI ] [ PubMed ] [ Google Scholar ]
- Di Chiara G., Imperato A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 85, 5274–5278 10.1073/pnas.85.14.5274 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Diana M., Brodie M., Muntoni A., Puddu M. C., Pillolla G., Steffensen S., Spiga S., Little H. J. (2003). Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol. Clin. Exp. Res. 27, 354–361 10.1097/01.ALC.0000057121.36127.19 [ DOI ] [ PubMed ] [ Google Scholar ]
- Diana M., Melis M., Muntoni A. L., Gessa G. L. (1998). Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc. Natl. Acad. Sci. U.S.A. 18, 10269–10273 10.1073/pnas.95.17.10269 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Diana M., Muntoni A. L., Pistis M., Melis M., Gessa G. L. (1999). Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur. J. Neurosci. 11, 1037–1041 10.1046/j.1460-9568.1999.00488.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Diana M., Pistis M., Carboni S., Gessa G. L., Rossetti Z. L. (1993). Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc. Natl. Acad. Sci. U.S.A. 90, 7966–7969 10.1073/pnas.90.17.7966 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Diana M., Pistis M., Muntoni A., Gessa G. L. (1996). Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: Evidence of protracted abstinence. Neuroscience 71, 411–415 10.1016/0306-4522(95)00482-3 [ DOI ] [ PubMed ] [ Google Scholar ]
- Diana M., Spiga S., Acquas E. (2006). Persistent and reversible morphine withdrawal-induced morphological changes in the nucleus accumbens. Ann. N. Y. Acad. Sci. 1074, 446–457 10.1196/annals.1369.045 [ DOI ] [ PubMed ] [ Google Scholar ]
- Erhardt A., Sillaber I., Welt T., Müller M. B., Singewald N., Keck M. E. (2004). Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology 29, 2074–2080 10.1038/sj.npp.1300493 [ DOI ] [ PubMed ] [ Google Scholar ]
- Feil J., Zangen A. (2010). Brain stimulation in the study and treatment of addiction. Neurosci. Biobehav. Rev. 34, 559–574 10.1016/j.neubiorev.2009.11.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- Frankle W. G., Laruelle M., Haber S. N. (2006). Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology 31, 1627–1636 10.1038/sj.npp.1300990 [ DOI ] [ PubMed ] [ Google Scholar ]
- Gariano R. F., Groves P. M. (1988). Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 462, 194–198 10.1016/0006-8993(88)90606-3 [ DOI ] [ PubMed ] [ Google Scholar ]
- Gonon F. (1988). Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24, 19–28 10.1016/0306-4522(88)90307-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- Groenewegen H. J., Berendse H. W., Meredith G. E., Haber S. N., Voorn P., Wolters J. G., Lohman A. H. M. (1991). “Functional anatomy of the ventral, limbic system-innervated striatum,” in The Mesolimbic Dopamine System: From Motivation to Action, eds Willner P., Scheel-Krüger J. (New York: Wiley; ), 19–59 [ Google Scholar ]
- Hyman S. E. (2007). The neurobiology of addiction: implications for voluntary control of behavior. Am. J. Bioeth. 7, 8–11 10.1080/15265160601063969 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalivas P. W., Hu X. T. (2006). Exciting inhibition in psychostimulation addiction. Trends Neurosci. 29, 610–616 10.1016/j.tins.2006.08.008 [ DOI ] [ PubMed ] [ Google Scholar ]
- Karreman M., Moghaddam B. (1996). The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J. Neurochem. 66, 589–598 10.1046/j.1471-4159.1996.66020589.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Keck M. E., Welt T., Müller M. B., Erhardt A., Ohl F., Toschi N., Holsboer F., Sillaber I. (2002). Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 43, 101–109 10.1016/S0028-3908(02)00069-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kenna G. A., Leggio L., Swift R. M. (2009). A safety and tolerability laboratory study of the combination of aripiprazole and topiramate in volunteers who drink alcohol. Hum. Psychopharmacol. 24, 465–472 10.1002/hup.1042 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kenny P. J., Chen S. A., Kitamura O., Markou A., Koob G. F. (2006). Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 26, 5894–5900 10.1523/JNEUROSCI.0740-06.2006 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kobayashi M., Pascual-Leone A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156 10.1016/S1474-4422(03)00321-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- Koob G. F., Kenneth Lloyd G., Mason B. J. (2009). Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat. Rev. Drug Discov. 8, 500–515 10.1038/nrd2828 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koob G. F., Volkow N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 10.1038/npp.2009.110 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kranzler H. R., Covault J., Pierucci-Lagha A., Chan G., Douglas K., Arias A. J., Oncken C. (2008). Effects of aripiprazole on subjective and physiological responses to alcohol. Alcohol. Clin. Exp. Res. 32, 573–579 10.1111/j.1530-0277.2007.00608.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lang N., Hasan A., Sueske E., Paulus W., Nitsche M. A. (2008). Cortical hypoexcitability in chronic smokers? A transcranial magnetic stimulation study. Neuropsychopharmacology 33, 2517–2523 10.1038/sj.npp.1301645 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lawford B. R., Young R. M., Rowell J. A., Qualichefski J., Fletcher B. H., Syndulko K., Ritchie T., Noble E. P. (1995). Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat. Med. 1, 337–341 10.1038/nm0495-337 [ DOI ] [ PubMed ] [ Google Scholar ]
- Leggio L., Cardone S., Ferrulli A., Kenna G. A., Diana M., Swift R. M., Addolorato G. (2010). Turning the clock ahead: potential preclinical and clinical neuropharmacological targets for alcohol dependence. Curr. Pharm. Des. 16, 2159–2181 10.2174/138161210791516413 [ DOI ] [ PubMed ] [ Google Scholar ]
- Manley L. D., Kuczenski R., Segal D. S., Young S. J., Groves P. M. (1992). Effects of frequency and pattern of medial forebrain bundle stimulation on caudate dialysate dopamine and serotonin. J. Neurochem. 58, 1491–1498 10.1111/j.1471-4159.1992.tb11369.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Martinez D., Gil R., Slifstein M., Hwang D. R., Huang Y., Perez A., Kegeles L., Talbot P., Evans S., Krystal J., Laruelle M., Abi-Dargham A. (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry 58, 779–786 10.1016/j.biopsych.2005.04.044 [ DOI ] [ PubMed ] [ Google Scholar ]
- Martinez D., Greene K., Broft A., Kumar D., Liu F., Narendran R., Slifstein M., Van Heertum R., Kleber H. D. (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am. J. Psychiatry 166, 1170–1177 10.1176/appi.ajp.2009.08121801 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martinez D., Kim J. H., Krystal J., Abi-Dargham A. (2007). Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin. N. Am. 17, 539–555 10.1016/j.nic.2007.07.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- Martinotti G., Di Nicola M., Di Giannantonio M., Janiri L. (2009). Aripiprazole in the treatment of patients with alcohol dependence: a double-blind, comparison trial vs. naltrexone. J. Psychopharmacol. 23, 123–129 10.1177/0269881108089596 [ DOI ] [ PubMed ] [ Google Scholar ]
- Melis M., Spiga S., Diana M. (2005). The dopamine hypothesis of drug addiction: hypodopaminergic state. Int. Rev. Neurobiol. 63, 101–154 10.1016/S0074-7742(05)63005-X [ DOI ] [ PubMed ] [ Google Scholar ]
- Meredith G. E., Baldo B. A., Andrezjewsky M. E., Kelley A. E. (2008). The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct. 213, 17–27 10.1007/s00429-008-0175-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mishra B. R., Nizamie S. H., Das B., Praharaj S. K. (2010). Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105, 49–55 10.1111/j.1360-0443.2009.02777.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Murase S., Grenhoff J., Chouvet G., Gonon F. G., Svensson T. H. (1993). Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci. Lett. 157, 53–56 10.1016/0304-3940(93)90641-W [ DOI ] [ PubMed ] [ Google Scholar ]
- Myrick H., Li X., Randall P. K., Henderson S., Voronin K., Anton R. F. (2010). The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J. Clin. Psychopharmacol. 30, 365–372 10.1097/JCP.0b013e3181e75cff [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Naranjo C. A., Dongier M., Bremner K. E. (1997). Long-acting injectable bromocriptine does not reduce relapse in alcoholics. Addiction 92, 969–978 10.1111/j.1360-0443.1997.tb02976.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Padberg F., George M. S. (2009). Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp. Neurol. 219, 2–13 10.1016/j.expneurol.2009.04.020 [ DOI ] [ PubMed ] [ Google Scholar ]
- Pistis M., Porcu G., Melis M., Diana M., Gessa G. L. (2001). Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur. J. Neurosci. 14, 96–102 10.1046/j.0953-816x.2001.01612.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Politi E., Fauci E., Santoro A., Smeraldi E. (2008). Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict. 17, 345–346 10.1080/10550490802139283 [ DOI ] [ PubMed ] [ Google Scholar ]
- Robinson T. E., Kolb B. (1997). Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 17, 8491–8497 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Robinson T. E., Kolb B. (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Suppl. 1), 33–46 10.1016/j.neuropharm.2004.06.025 [ DOI ] [ PubMed ] [ Google Scholar ]
- Rossetti Z. L., Melis F., Carboni S., Diana M., Gessa G. L. (1992). Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcohol. Clin. Exp. Res. 16, 529–532 10.1111/j.1530-0277.1992.tb01411.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Russo S. J., Dietz D. M., Dumitriu D., Morrison J. H., Malenka R. C., Nestler E. J. (2010). The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276 10.1016/j.tins.2010.02.002 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saletu B., Saletu M., Grünberger J., Frey R., Zatschek I., Mader R. (1990). On the treatment of the alcoholic organic brain syndrome with an alpha-adrenergic agonist modafinil: double-blind, placebo-controlled clinical, psychometric and neurophysiological studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 14, 195–214 10.1016/0278-5846(90)90101-L [ DOI ] [ PubMed ] [ Google Scholar ]
- Schulteis G., Markou A., Cole M., Koob G. F. (1995). Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U.S.A. 20, 5880–5884 10.1073/pnas.92.13.5880 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Semba J., Watanabe A., Kito S., Toru M. (1995). Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology 34, 785–791 10.1016/0028-3908(95)00059-F [ DOI ] [ PubMed ] [ Google Scholar ]
- Shapiro D. A., Renock S., Arrington E., Chiodo L. A., Liu L. X., Sibley D. R., Roth B. L., Mailman R. (2003). Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28, 1400–1411 10.1038/sj.npp.1300203 [ DOI ] [ PubMed ] [ Google Scholar ]
- Shearer J., Shanahan M., Darke S., Rodgers C., van Beek I., McKetin R., Mattick R. P. (2010). A cost-effectiveness analysis of modafinil therapy for psychostimulant dependence. Drug Alcohol Rev. 29, 235–242 10.1111/j.1465-3362.2009.00148.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Silvanto J., Cattaneo Z., Battelli L., Pascual-Leone A. (2008). Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J. Neurophysiol. 99, 2725–2730 10.1152/jn.01392.2007 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Silvanto J., Pascual-Leone A. (2008). State-dependency of transcranial magnetic stimulation. Brain Topogr. 21, 1–10 10.1007/s10548-008-0067-0 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sklair-Tavron L., Shi W. X., Lane S. B., Harris H. W., Bunney B. S., Nestler E. J. (1996). Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 1, 11202–11207 10.1073/pnas.93.20.11202 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spiga S., Lintas A., Migliore M., Diana M. (2010). Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addict Biol. 15, 266–276 10.1111/j.1369-1600.2010.00218.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Spiga S., Puddu M. C., Pisano M., Diana M. (2005). Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur. J. Neurosci. 22, 2332–2340 10.1111/j.1460-9568.2005.04416.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Spiga S., Serra G. P., Puddu M. C., Foddai M., Diana M. (2003). Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur. J. Neurosci. 17, 605–612 10.1046/j.1460-9568.2003.02435.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Strafella A. P., Paus T., Barrett J., Dagher A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21, RC157. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sundaresan K., Ziemann U., Stanley J., Boutros N. (2007). Cortical inhibition and excitation in abstinent cocaine-dependent patients: a transcranial magnetic stimulation study. Neuroreport 18, 289–292 10.1097/WNR.0b013e3280143cf0 [ DOI ] [ PubMed ] [ Google Scholar ]
- Swift R. M. (2010). Medications acting on the dopaminergic system in the treatment of alcoholic patients. Curr. Pharm. Des. 16, 2136–2140 10.2174/138161210791516323 [ DOI ] [ PubMed ] [ Google Scholar ]
- Taber M. T., Das S., Fibiger H. C. (1995). Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J. Neurochem. 65, 1407–1410 10.1046/j.1471-4159.1995.65031407.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Thanos P. K., Taintor N. B., Rivera S. N., Umegaki H., Ikari H., Roth G., Ingram D. K., Hitzemann R., Fowler J. S., Gatley S. J., Wang G. J., Volkow N. D. (2004). DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol. Clin. Exp. Res. 28, 720–728 10.1097/01.ALC.0000125270.30501.08 [ DOI ] [ PubMed ] [ Google Scholar ]
- Thanos P. K., Volkow N. D., Freimuth P., Umegaki H., Ikari H., Roth G., Ingram D. K., Hitzemann R. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103 10.1046/j.1471-4159.2001.00492.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Urban N. B., Kegeles L. S., Slifstein M., Xu X., Martinez D., Sakr E., Castillo F., Moadel T., O’Malley S. S., Krystal J. H., Abi-Dargham A. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C] raclopride. Biol. Psychiatry 68, 689–696 10.1016/j.biopsych.2010.06.005 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Volkow N. D., Wang G. J., Begleiter H., Porjesz B., Fowler J. S., Telang F., Wong C., Ma Y., Logan J., Goldstein R., Alexoff D., Thanos P. K. (2006). High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch. Gen. Psychiatry 63, 999–1008 10.1001/archpsyc.63.9.999 [ DOI ] [ PubMed ] [ Google Scholar ]
- Volkow N. D., Wang G. J., Fowler J. S., Logan J., Hitzemann R., Ding Y. S., Pappas N., Shea C., Piscani K. (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 20, 1594–1598 10.1111/j.1530-0277.1996.tb05936.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Volkow N. D., Wang G. J., Telang F., Fowler J. S., Logan J., Jayne M., Ma Y., Pradhan K., Wong C. (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci. 27, 12700–12706 10.1523/JNEUROSCI.3371-07.2007 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Voronin K., Randall P., Myrick H., Anton R. (2008). Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm-possible influence of self-control. Alcohol. Clin. Exp. Res. 32, 1954–1961 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weiss F., Parsons L. H., Schulteis G., Hyytia P., Lorang M. T., Bloom F. E., Koob G. F. (1996). Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 16, 3474–3485 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wise R. A. (1980). Action of drugs of abuse on brain reward systems. Pharmacol. Biochem. Behav. 13(Suppl. 1), 213–223 10.1016/S0091-3057(80)80033-5 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wise R. A. (1987). The role of reward pathways in the development of drug dependence. Pharmacol. Ther. 35, 227–263 10.1016/0163-7258(87)90108-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Yeomans J. S. (1989). Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci. Biobehav. Rev. 13, 91–98 10.1016/S0149-7634(89)80016-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- Yeomans J. S., Mathur A., Tampakeras M. (1993). Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav. Neurosci. 107, 1077–1087 10.1037/0735-7044.107.4.596 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zangen A., Hyodo K. (2002). Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 13, 2401–2405 10.1097/00001756-200212200-00005 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (515.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
“Gateway hypothesis” and early drug use: Additional findings from tracking a population-based sample of adolescents to adulthood
Stephen nkansah-amankra, mark minelli.
- Author information
- Article notes
- Copyright and License information
Corresponding author at: Sam Houston State University, Department of Population Health, College of Health Sciences, 411 CHS Building, 77941, Huntsville, TX, United States.Sam Houston State UniversityDepartment of Population HealthCollege of Health Sciences411 CHS Building, 77941HuntsvilleTXUnited States
Dr. Nkansah-Amankra is the Primary Author of this article . He designed the study, conducted the statistical analysis of the data, developed narrative and served as the team leader for this research study.
Dr. Minelli was the secondary author of this article. He contributed to the narrative sections, editing and was active in team research meetings to discuss the study methods and findings.
Received 2016 Feb 2; Revised 2016 May 4; Accepted 2016 May 16; Collection date 2016 Dec.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
To evaluate the consistency of the relationship between early drug use in adolescence and illegal drug use in adulthood as proposed in the “gateway theory” and to determine whether pre-existing depressive symptoms modifies this relationship. We used contractual data from the National Longitudinal Study of Adolescent to Adult health data spanning a 14 year period. We assessed the relationship between gateway drugs at baseline (age 11–20 years) and drug use in adulthood using generalized estimating equation (GEE) regression models. Gateways drugs used in early adolescence were significantly associated with marijuana use, illegal drugs and cocaine in older adolescence, but over time these relationships were not consistent in adulthood. Changes in the pattern of psychoactive drug use were important predictors of drug use in adulthood. A history of higher depressive symptoms was associated with higher frequencies of psychoactive drug use over time. Users of mental health services in adolescence were less likely to use drugs in older adolescence and in adulthood. Relationships between early drug use and later drug use in adulthood cannot be solely explained by the gateway hypothesis. Collectively, adolescent drug prevention and treatment programs should apply theory-based and evidence-proven multisectoral intervention strategies rather than providing a brief counseling on individual's behaviors. This evidence should include understanding that changes in behavior should involve broader analyses of the underlying social context for drug use and in particular the role of the community social norms in driving a group's behaviors.
Keywords: Gateway theory, Gateway drugs, Depressive symptoms, Early drug use, Young people
Evidence shows early use of legal drugs increase risks of illicit drug progression.
It is unclear if adolescents at different stages, experience drug use in adulthood.
We used a nationally-based longitudinal data to evaluate these relationships.
Early drug initiation increased risks of drug progression but not in adulthood.
GH might be inadequate to explain drug use over time.
1. Introduction
The concept of “gateway hypothesis” has been studied since the 1970s ( Kandel, 1975 , Kandel and Faust, 1975 ) as the theory suggests that an adolescent's early experimentation with alcohol or tobacco or cannabis escalates to more addictive illicit drugs later in adulthood ( Lynskey et al., 2003 ). Most commonly used illicit substances include heroin/opioids, cocaine and or amphetamines and their designer drug analogs, considered illegal by the criminal justice system in the United States and other jurisdictions. Early onset or drug experimentation has been elaborated and characterized in distinct pathways in the substance abuse and dependence literature. Overall, the theory has had mixed results showing both a link or sequence of licit drug use to illicit drug use ( Guxens et al., 2007 , Guxens et al., 2007 , Korhoene et al., 2010 , Lessem et al., 2006 , Mayet et al., 2012 ) and no association ( Mackesy-Amiti et al., 1997 , Golub and Johnson, 1994 ).
Although the concept has also been a subject of considerable scholarly and political discourse in western societies, a review of the literature often shows less consensus on research and policy relevance among investigators. An earlier series of studies ( Kandel, 1975 , Kandel and Faust, 1975 , Kandel et al., 1992 ) among adolescents showed the existence of a significant and a clearly defined sequence of drug use onset starting with licit substances (alcohol, cigarette) and progression to illicit drugs (cocaine, marijuana, methamphetamine, and heroin) through adulthood. Recently, Kandel and Kandel (2014) , have demonstrated the GH with animal studies and their findings showed that use of one drug enhances effects of the other drugs — a process hypothesized as due to the priming of the neural circuitry of the brain. Fergusson et al. (2006) analyzed a population-based data on cannabis use and progression to other illicit drugs among a 25-year longitudinal study of 1265 birth cohorts from Christchurch, New Zealand. The investigators found strong evidence for causal model of GH, in which earlier use of cannabis was hypothesized as causing increased use of other illicit drugs. In addition, numerous prior studies have failed to disconfirm causal links of gateway effects in human populations ( Kandel, 2002 , Gundy and Rebellon, 2010 , Morral et al., 2002 ). However, a cross-country comparison of the GH by Degenhardt et al. (2010) found background prevalence of the gateway drugs or their availability as the major driving factor for drug use progression across countries analyzed. Another study analyzing a sample of adolescents from South Florida showed that marijuana gateway effect is contingent on context of age ( Gundy and Rebellon, 2010 ). In spite of these, it is still unclear the extent to which a cohort of adolescents at different developmental stages experience gateway drug use (tobacco, alcohol, marijuana) as determinants of later illicit drug use during and prior to adulthood.
Consistent with the theory, research in the substance abuse literature has focused on age of onset of substance used as a proximate determinant of future drug use and dependence in adulthood ( Chen et al., 2009 , Behrendt et al., 2009 , Trenz et al., 2012 , Mayet et al., 2012 ). Trenz et al. (2012) found early onset of alcohol at age 15 but not cigarette or marijuana among adolescents as a risk factor for injection drug use in adulthood. An earlier study by Chen et al. (2009) , reported clinical manifestation of drug dependence and other health problems among adolescents' early onset (11–17 years) of drug use compared with adult (18 +) recent users. Likewise, Lynskey et al., report that among discordant twins, individuals starting cannabis use before age 17 were at increased risk of illicit drug use and drug dependence. Mayet et al. (2012) found that among 17-year olds participating in a military exercise in France, initiating one drug increased the risk of initiating the other drug use, consistent with the gateway theory. However, the risk of an experimenter becoming a daily user of tobacco was higher for initial tobacco users than cannabis. A recent follow-up study by Mayet et al. (2012) found daily tobacco use among adolescents as strongly associated with cannabis initiation and other illicit drugs. However, deviations to these patterns of drug use have been also observed in studies and hypothesized to be linked to an underlying mental health condition of respondents ( Degenhardt et al., 2010 ). One study of cross national comparisons of 17 countries found prior drug use and age of onset as the most dominant factors determining drug dependence ( Degenhardt et al., 2010 ). In addition, there were considerable variations of early onset of drug use among similar age cohorts in different countries. Unfortunately the prospective relationship between early drug onset in adolescence and drug use transition in adulthood was not evaluated.
Although the evidence suggests that substance use dependence may also occur with the initial drug experimentation of commonly available legal substances ( Kirby and Barry, 2012 ), continual use over time may increase the likelihood of developing risks for substance use disorders ( Deza, 2015 ) and other substance-related illnesses. In this regard, Midanik et al. (2007) , reported that simultaneous alcohol and cannabis use was related to increased prevalence rates of other social consequences including problem behaviors, alcohol dependence and depression. A twin-study of young women by Agrawal et al. (2009) , found that women's initial use of tobacco and cannabis simultaneously was more likely to experience higher rates of DSM IV cannabis abuse but not dependence. The possibility of other addictive drugs (codeine and other prescription drugs) and substances (hallucinogens, inhalants, ecstasy, amphetamines) resulting in poor health sequelae due to initial drug experimentation has been noted in some studies ( Fairman, 2015 , Deza, 2015 ). This is important considering the extent of initial substance use or use combinations could lead to more widespread illegal drugs or addictive behaviors over time, replications of these findings in a nationally representative sample of adolescents transitioning to adulthood are needed to understand the continuum of progression of drug use over the life course (from adolescence to adulthood).
Despite a great uncertainty about the gateway theory, with few exceptions ( Behrendt et al., 2009 , Fergusson et al., 2006 ) there has been remarkably less rigorous empirical assessment with a population-based sample, prospectively assessing the impact of early drug use on later drug use as well as related mental health conditions (depressive symptoms). Data from longitudinal studies will allow for additional questions to be explored including how changes in drug use over time from early adolescence to adulthood might be related to earlier onset of drug use and a pattern of individual drug use trajectories during transition to adulthood. Sequence of drug initiation may be due to several factors including effects of one drug use on another, familial and demographic and psychosocial characteristics or a combination of different factors ( Guerra et al., 2000 ). In addition most of these studies did not control for current substance use, a factor which is an important determinant for fully understanding how earlier drug use or non-drug use may change over time from adolescence to young adulthood.
The aim of this study is to evaluate the impact of early substance use on later illicit drug use while accounting for concurrent drug use over a relatively longer period among a cohort of adolescents transitioning to adulthood, and to determine the extent to which these relationships conform to the GH. Our analyses here examine the relationship between early gateway drug use and future illicit drug use among a cohort of adolescents, and to determine whether causal or non-causal inferences are warranted. We anticipate gateway drug use among our sample to escalate to illicit drug use in adulthood and we expect this relationship to be non-causal. Our hypothesis is that any gateway relationship in adulthood reflects spurious effects of underlying depressive symptoms and age as well as modifying influences of these factors (age and depressive symptoms). Second, we were also interested in investigating the relationship between early drug use onset in adolescence and substance use in adulthood taking into account the existing concurrent mental health status of individuals at each developmental stage. To the extent that gateway associations to illicit drug use among older adolescence or adulthood is causal, we evaluate the stage (older adolescence or adulthood) at which this relationship is likely to significant and if it has short or long term effect in adulthood. We hypothesize that the gateway relationship to adult drug use is transient only among older adolescence and the relationship is modified by depressive symptoms reported in older adolescence.
2. Materials and methods
The study sample was generated from the National Longitudinal Study of Adolescent to Adult Health (Add Health). The Add Health study is a national longitudinal survey of school-based representative sample of students in grades 7–12 in 1994 academic year in the United States. The cohort from this study were selected and interviewed in 1994–95 school year as in-home samples (79% response rate as a proportion of selected in-home sample) for Wave I ( N = 20,745). This sample has been followed over time with three further in-home interviews in 1996 Wave II (11–21 years, response rate 88.6%; N = 14,738), Wave III 2001–2002 (aged 18–26 years, response rate 77.4%; N = 15,197) with the most recent data occurring in Wave IV 2007–08 (aged 24–32 years, response rate 80.3%; N = 15,701). Our analysis used the restricted Add Health datasets, and the detailed description of the study design is found in other publications ( Bearman et al., 1997 , Nkansah-Amankra et al., 2012 ). In this analysis, we a used a total sample of 11,194 observations with complete survey weights and information across all four waves. The study was approved by the Institutional Review Board (IRB) of Central Michigan University.
2.1. Outcome measures
We used the following illegal substances from Waves II to IV as our outcome measures: marijuana, illicit drugs (Add Health instrument gathered information specifically on heroin, amphetamines, LSD, PCP, ecstasy, speed, ice to assess the illicit drugs variable) and cocaine. At each wave of data collection, participants were asked if they had used each of the above substances in the past 30 days. We created a two-level outcome measure for each psychoactive substance used from older adolescence (Wave 2) to adulthood (Waves 3 and 4). Respondents reporting not using a substance served as the reference.
2.2. Exposure
The exposure variables of interest were the three known “gateway substances” used in early adolescence (as measured in Wave I): tobacco, alcohol and marijuana. These were measured with two items, one stating if respondents had used any of these substances and the follow-up question asking the age at which they started using each substance for the first time. These two items were combined to create the age of use for the following substances: tobacco, marijuana, and alcohol as ≤ 10, 11–15, and 16–18 consistent with previous investigations ( Tarter et al., 2012 ). We were also interested in examining age of onset of illicit drugs and Cocaine use in this age groups (≤ 10, 11–15, and 16–18). In a separate analysis we specifically examined changes in the gateway drug exposures in adolescence and changes in the pattern of illegal drug use in adulthood.
2.3. Covariates
These include age ranges 12–19, 18–26 and 26–32 (at respective Waves 2, 3 and 4), race (Black, White and Hispanics) and current substance used (marijuana, illegal drugs and cocaine) at a particular survey period to control for potential influences on early drug use and later substance use. We used the Center for Epidemiologic Studies of Depression (CES-D) scale as the measure of depressive symptoms and an indicator of mental health condition ( Radloff, 1977 ), but Waves III and IV used only 9-items. Each response from the original item scale was coded as 0–3 (0 = rarely or none of the time, 3 = most of the time), and four-positively formulated items in the original scale were reverse coded to enhance comparability in calculating the summative score. Higher CES-D scores indicate negative emotions or negative affect. The consistency of the 9-item scale in measuring depressive symptomatology has been affirmed in numerous studies ( Levine, 2013 , Zhang et al., 2012 ). Across all four waves we created comparable 9-item CES-D scales to assess depressive symptoms. Response to mental using the mental health services item in the instrument was used as a measure of access mental health services across waves of data collection.
3. Statistical analysis
All statistical analyses were estimated using SAS Callable SUDAAN version 9.0 (SAS Institute, Cary, NC and RTI, Cary, NC) to account for sampling weights and other survey characteristics in determining the standard errors. Statistical significance for unadjusted comparisons was assessed by using Rao Scott χ 2 tests. We evaluated cohort-specific analysis to determine the relationship between initial drug intake and later illicit drug progression across different waves of data collection. We estimated the odds ratios (ORs) and 95% confidence intervals (CIs) using the baseline drug use and later drug progression using the category of non-users as the reference groups. These models were estimated with generalized estimating equation (GEE) for repeated measures using cumulative logit link function and simultaneously adjusting for multiple covariates. To model the relationship between early substance used and later substance use as exhibiting a change over time, the variation in early exposures and changes in later drug use escalation were examined. Analyses were 2-sided and p-values < .05 were considered statistically significant.
Table 1 shows the distribution of baseline mean age, age at first substance use and other socio-demographic characteristics of Add Health participants, 1994/95. The age group ≤ 15 years consistently reported higher percent frequency distribution of substance use in Wave I.
Baseline characteristics of Add Health study participants according to early drug use, 1994–2008.
Unweighted sample distribution.
Weighted percentage distribution.
This variable refers to the use of LSD, amphetamines or heroin and their derivatives and any other illicit more active psychoactive substance.
Used of mental health services (No = nonuse of mental health services; Yes = reported using mental health services).
Fig. 1 shows a box and whisker plot of depressive symptomatology (median, 25th–75th percentiles) participants reporting using separately each gateway drug from Waves I to IV. These plots reveal strong correlations among cigarette smoking (60.8%) or alcohol use (38.5%) and reporting of higher depressive symptoms over time. That is, gateway substance users are over time more likely to report depressive symptoms (as measured with CES-D). However, both cigarette smokers and alcohol users are over time more likely to report relatively higher depressive symptoms than marijuana users.
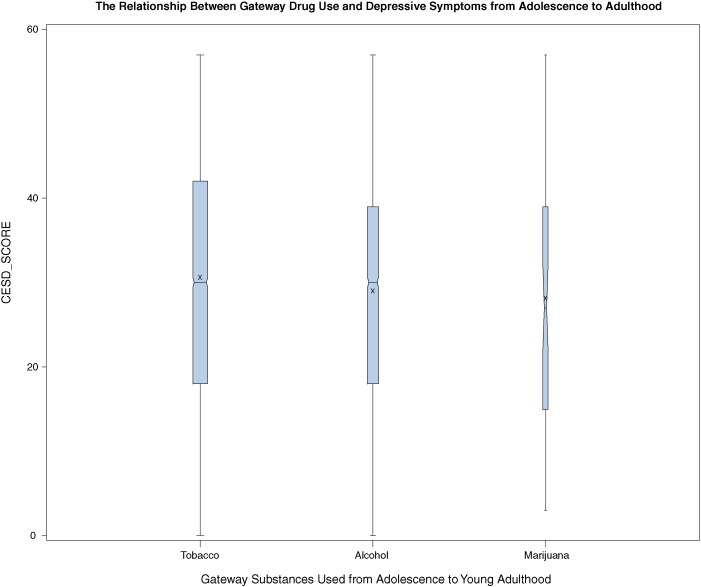
The Relationship between Gateway Drugs Used and Depressive Symptoms from Adolescence to Adulthood.
Associations of baseline characteristics with psychoactive substance use over the period of follow-up data are shown in Table 2 . Age group 11–15 years or below reported the highest frequency of drug use over time, compared to other age groups. More than three-quarters of the sample using alcohol in Wave I (11–15 years) reported using all types of illicit drugs over time, but a little more than half of tobacco in Wave I used different illicit drugs. More than half of marijuana users in this age category used marijuana a year later but the usage of other illicit drugs was not consistent over time. Overall, illegal drugs and cocaine in particular were least likely to be used from adolescence to adulthood.
Distribution of population characteristics by early psychoactive drug use a among Add Health participants, baseline in 1994, follow-up in 1996–2008.
Add Health = National Longitudinal of Adolescent to Adult Health; CES-D = The Center for Epidemiologic Studies Depression Scale.
n = Sample distribution of main variables and other covariates. Figure in parentheses refers to unweighted distribution and % †† describes weighted per cent distribution.
Significant differences between groups at α = 0.05, tested using χ 2 tests for categorical variables and analysis of variance for continuous variables.
Per cent are weighted to account for sampling weights.
Categories shown above are not mutually exclusive.
Illegal drugs include any type of illicit drug such as LSD, PCP, ecstasy, mushrooms, speed, ice, heroin or pills without a doctor's prescription.
Mean age of respective drug used across different wave of data collection.
Relationships among various psychoactive substance uses with the baseline age of substance use measured at 3 different survey waves are shown in Table 3 . The first three columns of Table 3 show odds ratio (OR) estimates and corresponding 95% confidence intervals (95% CI) for predicting drug use and mental health services access among older adolescents. Tobacco, marijuana, any illegal drugs and age at cocaine use in adolescence was significantly associated with marijuana use, illegal drugs and cocaine in older adolescence, but over time these relationships were not consistent as expected from the gateway hypothesis. Using marijuana at baseline appeared to be consistently associated with increased likelihood of using other psychoactive substances in late adolescence and in young adulthood compared with non-users. Alcohol use in Wave I was less likely to be associated with any psychoactive substances in older adolescence and over time, but tobacco use greatly increased the odds of using marijuana, cocaine and illegal drugs in older adolescence. Cigarette smoking greatly increased the odds of using cocaine in early adulthood among all age groups reporting smoking in Wave I.
Adjusted odds ratios of all later psychoactive substance use (versus non-use) according to baseline characteristics among National Longitudinal Study of Adolescent to Adult Health (Add Health) participants, baseline in 1994, follow-up in 1996–2008.
Abbreviations: Add Health is National Longitudinal Study of Adolescent to Adult Health; OR, odds ratio, 95% CI: 95% confidence interval.
Multivariable analyses adjusted for demographic characteristics, access to mental health, and previous wave of psychoactive drug use.
Statistically significant differences between groups at α = 0.05. Bold-faced indicate statistically significant differences.
The pattern consistent with the gateway hypothesis was not present across the waves of data collection in to adulthood. However, among the three gateway substances initiated in early adolescence marijuana appeared somehow to have a greater and consistent effect in determining the likelihood of using other psychoactive substances over time in adulthood.
There were significant interactions between the three gateway drugs and depressive symptoms for marijuana, illegal drugs and cocaine used in older adolescence and adulthoods (results not shown). Age groups 11–15 years smoking cigarette in Wave 1 and reporting high depressive symptoms (in Wave I) increased the odds of smoking marijuana in older adolescence (OR = 1.37; 95% CI = 1.08, 1.74) and young adulthood (OR = 1.54; 1.10, 2.16), whereas age groups 16–18 smoking cigarette in Wave I and reporting high depressive symptoms in Wave 1 were at higher odds for illegal drug use in older adolescence (OR = 10.08; 95% CI = 1.59, 63.96).
Table 4 shows results from changes in the use of three gateway drugs in adolescence and the likelihood of using illegal substances in adulthood. Controlling for all potential confounders (race, age and current drug use) persistent smoking in adolescence was associated with increased odds of marijuana use in early adulthood, and marijuana (OR = 1.47; 95% CI = 1.17, 1.83), illegal drugs (OR = 3.28; 95% CI = 2.73, 3.94) and cocaine (OR = 3.70; 95% CI = 3.09, 4.44) in young adulthood. Only heavy alcohol users were at increased odds of using marijuana in early adulthood and higher odds of using illegal drugs and cocaine in young adulthood.
Adjusted odds ratios ¶ and corresponding 95% confidence intervals of changes occurring in using drugs in early or young adulthood among respondents participating in the National Longitudinal Study of Adolescent Health (Add Health), 1994–2008.
Abbreviations: CI = confidence interval; OR, odds ratio; Add Health.
CES-D is Center for Epidemiologic Study of Depression Scale.
§ Initial drug use refers to use of tobacco, alcohol, marijuana (gateway drugs) at baseline of the study, in the early adolescence. Reference group is non-users of a particular gateway drug at the respective developmental stage.
Adjusted for the following covariates at different exposures: age, gender, race,
Young adulthood (age groups 19-23 years).
Older adulthood (age groups 24-33 years).
We next investigated whether the observed effects resulting from the changes in the gateway drug use in adolescence and depressive symptoms (CES-D) were consistent determinants of illegal drug use in adulthood. Non-smokers in Waves 1 and 2 and reporting high depressive symptoms in Wave 3 had 1.5 times the odds of smoking marijuana in early (OR = 1.52: 95% CI = 1.11, 2.08) and young (OR = 1.55 95% CI = 1.11, 2.16) adulthoods but lower risk of using illegal drugs in early adulthood (OR = 0.29, 95% CI = 0.13, 0.66). Current smoking status in both waves and reporting elevated depressive symptoms in Wave 2 increases the odds of using illegal drugs in early adulthood (OR = 2.22, 95% CI = 1.12, 4.40), or smoking marijuana in young adulthood (2.32 (95% CI = 1.52, 3.56). But those quitting smoking in Wave 2 and reporting high depressive symptoms in Wave 2 had more than 24 times the odds of using illegal drugs in early adulthood (OR = 24.51, 95% CI = 1.87, 322.02). Individuals taking alcohol either in Wave 1 or Wave 2 (fluctuating drinkers) and reporting low depressive symptoms in Wave 1 were at increased odds of smoking marijuana in Wave 3 (OR = 4.41; 95% CI = 1.12, 17.34).
5. Discussion
In this prospective cohort study, early use of psychoactive substances — smoking cigarette, alcohol and illegal drugs (as earlier defined) was associated with increased likelihood of using marijuana, illegal drugs and to a large extent cocaine use in older adolescence. First, early exposure to marijuana and illegal substances was also positively associated with illegal substance and cocaine use in young adulthood. Second, cocaine use in early adolescent appeared uniquely to have ‘a long reach’ in later cocaine use in young adulthood. However, over time from adolescence to adulthood, we did not observe a pattern where early exposure to commonly known psychoactive substances — cigarette smoking or alcohol escalates to marijuana use or illegal psychoactive substances as posited by the ‘gateway theory.’ Finally, interactions between the gateway drugs and reporting high depressive symptoms in adolescence or adulthood were associated with increased use of marijuana, illegal drugs and cocaine in early or young adulthood.
Our finding that early exposure to cigarette smoking and alcohol use was positively associated with later (almost 10.4 months) use of illegal psychoactive substances among older adolescence is consistent with numerous studies on the gateway hypothesis ( Kandel, 2002 , Agrawal et al., 2009 , Mayet et al., 2012 ). However, our findings showed that over a relatively longer period of time (from adolescence to adulthood), early use of marijuana and other illegal drugs rather than tobacco or alcohol greatly increases the likelihood of using cocaine and other illegal drugs. A co-twin study in Australia found early cannabis use as a consistent predictor for other psychoactive substance use and in development of drug dependence ( Lynskey et al., 2003 ).
Contrary to our findings, Tarter et al. (2012) did not find early drug use of gateway drugs (tobacco, alcohol) as predicting marijuana and other illicit drug use. Participants in this study started using marijuana before tobacco or alcohol. However, this prior finding reflects ease of access to marijuana or other commonly available drugs rather than a defined pattern of drug escalation within a framework of causality. This needs further investigations.
Our data reveal that early use of psychoactive substances is associated with increased likelihood of using further illicit substances during adolescent period, but effects of these substances on later illicit drug use are inconsistent. However, early use of marijuana also appears to more readily ‘open the gate’ towards later use of other illicit substances. These findings are remarkable in view of the current debates on legalizing marijuana for recreational and medical uses, and the fact that our sample is population-based. Clearly, marijuana use in early adolescence enhances increased likelihood of continuing use of other psychoactive substances, and may be further compromised by underlying mental health condition. Existing drug policy and intervention programs have placed more emphases on tobacco, alcohol as ‘gateway’ drugs to later illicit drugs, but our findings suggest that attention should equally be placed on marijuana and other psychoactive substances in some population groups particularly in the age groups ≤ 15.
Our findings also reveal that it is not solely early exposure to psychoactive substances that matters for later drug use, but also the timing of the exposure to these ‘gateway drugs.’ For some illegal drug use outcomes, particularly those related to marijuana use, alcohol and to some extent tobacco exposures in adolescence may be especially harmful in young adulthood. Both heavy and moderate users of alcohol as well as adolescents using marijuana (of different amounts) in Waves I and II were at increased odds of using illegal drugs and cocaine in young adulthood. The construct of “gateway theory” or GH has some heuristic and intuitive appeal to the academics, policy makers and the general public. The idea of gateway substance use among adolescents actually assumes that once consumption of psychoactive substance is initiated the trend is to escalate and suggests that adolescent behaviors are immutable. Even though this is appealing, the idea is inconsistent with age-related reductions in drug use observed in the human development phenomenon described as ‘maturing out’ ( Lee et al., 2013 ) during emergent adulthood ( Littlefield et al., 2009 ).
Strengths of this study include the length (≥ 14 years) of the Add Health data and the sampling procedure used allowed prospective analyses of variations in different psychoactive drugs used from early adolescence to young adulthood. Availability and inclusion of current drugs used in statistical models across each wave enabled ascertainment of effects of earlier drugs used on current illicit drugs (while controlling for previous drugs). Given the relatively large sample size, we were able to model changes in drug use during adolescence and likelihood of using other drugs in adulthood — a feature that has not been applied in numerous studies. Another unique contribution of this study was analyses of interactive effects among early drug use, depressive symptoms (mental illness) on the risk of later drug use in adulthood, features which were not available in other studies. However, our study has some limitations. First, the taxonomy used in classifying drugs is purely based on the legal status, and social acceptability, not necessarily on the basis of the inherent harm each is likely to cause. Ideally, classifying drugs on dimensions of harmfulness or increased likelihood of addiction and potential size of a threat to the individual and the larger society should be the focus of future investigations. Second, we could not measure amounts of early psychoactive substances that precipitated the use of ‘hard and illicit’ drugs over time. It is almost impossible to evaluate or predict with certainty the quantity of each psychoactive substance(s) needed to achieve these changes. Third, our study could not also examine other psychological and pathological variables associated with early or continuing drug use. In particular, it is not clear whether individuals using marijuana to reduce anxiety in social circumstances such as to avoid certain negative social affects ( Buckner et al., 2011 ). Fourth, if unobserved environmental or genetic factors are associated with early psychoactive substance use and follow-ups, then our model estimations might be biased, and in that case it might be inappropriate to assume that later drug use is solely attributable to early drug use. Finally, historical and secular trends occurring in the use of licit or illicit substances in the country might suggest that describing substance use in terms of onset might be too simplistic because such an account might not consider substance use history over time.
6. Conclusion
In conclusion, this study did not find that the proportion of the population using alcohol, tobacco or marijuana in early adolescence showed patterns of increasing use of marijuana, illegal drugs or cocaine according to the length of follow-up (approximately 14 years). These findings suggest that adolescent drug prevention and treatment programs should apply proven multi-sectoral prevention strategies rather than providing brief counseling methods only on individual behaviors. While individual behavior change is desirable, a focus on the individual may be inconsequential compared to radical changes that may need to be made at the broader societal contexts. In addition, such efforts must not only focus on licit substances but include marijuana and assess the underlying mental illness predisposing young people to early drug use. In particular adolescents' recreational use of marijuana needs to be discouraged at the earliest age and medical marijuana use must have strict adherence to treatment regimen.
- Agrawal A., Lynskey M.T., Madden P.A.F., Pergadia M.L., Bulchoz K.K., Heath A.C. Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug Alcohol Depend. 2009;101:8–12. doi: 10.1016/j.drugalcdep.2008.10.019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bearman P.S., Jones J., Udry J.R. 1997. The National Longitudinal Study of Adolescent Health: Research Design. Available via internet at: http://www.cpc.unc.edu/addhealth. [ DOI ] [ PubMed ] [ Google Scholar ]
- Behrendt S., Wittchen H.-U., Höfler L.R., Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- Buckner J.D., Silgado J., Schmidt N.B. Marijuana craving during a public speaking challenge: understanding marijuana use vulnerability among women and those with social anxiety disorder. J. Behav. Ther. Exp. Psychiatry. 2011;42:104–110. doi: 10.1016/j.jbtep.2010.07.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen C.-Y., Storr C.L., Anthony J.C. Early-onset drug use and risk for drug dependence problems. Addict. Behav. 2009;34:319–322. doi: 10.1016/j.addbeh.2008.10.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Degenhardt L., Dierker L., Chiu W.T. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order initiation of drug use among participants in the WHO World Mental Surveys. Drug Alcohol Depend. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deza M. Is there a stepping stone effect in drug use? Separating state dependence from unobserved heterogeneity within and between illicit drugs. J. Econ. 2015;184:193–207. [ Google Scholar ]
- Fairman B.J. Cannabis problem experiences among users of the tobacco-cannabis combination known as blunts. Drug Alcohol Depend. 2015;150:77–84. doi: 10.1016/j.drugalcdep.2015.02.014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fergusson D.M., Boden J.M., Horwood L.J. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Golub A., Johnson B. The shifting importance al alcohol and marijuana as gateway substances among serious drug abusers. J. Alcohol. 1994;55:607–614. doi: 10.15288/jsa.1994.55.607. [ DOI ] [ PubMed ] [ Google Scholar ]
- Guerra L.S., Romano P.S., Samuels S.J., Kass P.H. Ethnic differences in adolescent substance initiation sequences. Arch. Pediatr. Adolesc. Med. 2000;154:1089–1095. doi: 10.1001/archpedi.154.11.1089. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gundy K.V., Rebellon C.J. A life course perspective on the "Gateway Hypothesis. J. Health Soc. Behav. 2010;51(3):244–259. doi: 10.1177/0022146510378238. [ DOI ] [ PubMed ] [ Google Scholar ]
- Guxens M., Nebot M., Ariza C. Age and sex differences in factors associated with the onset of cannabis use: a cohort study. Drug Alcohol Depend. 2007;88:234–243. doi: 10.1016/j.drugalcdep.2006.10.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kandel D. Cambridge University Press; Cambridge, England: 2002. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. [ Google Scholar ]
- Kandel D.P., Faust R. Sequence and stages in patterns of adolescent drug use. Arch. Gen. Psychiatry. 1975;32:923–932. doi: 10.1001/archpsyc.1975.01760250115013. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kandel E.R., Kandel D.B. A molecular basis for nicotine as a gateway drug. N. Engl. J. Med. 2014;371(10):932–943. doi: 10.1056/NEJMsa1405092. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kandel D.B., Yamaguchi K., Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J. Stud. Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kirby T., Barry A.E. Alcohol as a gateway drug: a study of U.S. 12th graders. J. Sch. Health. 2012;82:371–379. doi: 10.1111/j.1746-1561.2012.00712.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Korhoene T., Prince van Leeuven A., Rejneveld S.A., Ormel J., Verhulst F.C., Huizinc A.C. Externalizing behavior problems and cigarette smoking as predictors of cannabis use: the TRAILS study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:61–69. doi: 10.1097/00004583-201001000-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lee M.R., Chassin L., Villalta I.K. Maturing out of alcohol involvement: transitions in latent drinking statuses from late adolescence to adulthood. Dev. Psychopathol. 2013;25:1137–1153. doi: 10.1017/S0954579413000424. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lessem J., Hopfer C., Haberstick B. Relationship between adolescent marijuana use and young adult illicit drug use. Behav. Genet. 2006;36:4. doi: 10.1007/s10519-006-9064-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Levine S.Z. Evaluating the seven-item Center for Epidemiologic Studies Depression Scale short form: a longitudinal US community study. Soc. Psychiatry Psychiatr. Epidemiol. 2013;48:1519–1526. doi: 10.1007/s00127-012-0650-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Littlefield A.K., Sher K.J., Wood P.K. Is “maturing out” of problematic alcohol involvement related to personality change? J. Abnorm. Psychol. 2009;118:360–374. doi: 10.1037/a0015125. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lynskey M.T., Heath A.C., Bucholz K.T. Escalation of drug use in early-onset cannabis users vs co-twin controls. J. Am. Med. Assoc. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mackesy-Amiti M.E., Frendrich M., Goldstein P.J. Sequence of drug use among serious drug users: typical vs atypical progression. Drug Alcohol Depend. 1997;45:185–196. doi: 10.1016/s0376-8716(97)00032-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mayet A., Legleye S., Falissard B., Chau N. Cannabis use stages as predictors of subsequent initiation with other illicit drugs among French adolescents: use of a multi-state model. Addict. Behav. 2012;37:60–166. doi: 10.1016/j.addbeh.2011.09.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- Midanik L.T., Tam T.W., Weisner C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 2007;90:72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morral A.R., McCaffrey D.F., Paddock S.M. Reassessing marijuana gateway effect. Addiction. 2002;97:1493–1504. doi: 10.1046/j.1360-0443.2002.00280.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nkansah-Amankra S., Diedhiou A., Agbanu S.K. A longitudinal evaluation of religiosity and psychosocial determinants of suicidal behaviors among a population-based sample in the United States. J. Affect. Disord. 2012;139:40–51. doi: 10.1016/j.jad.2011.12.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- Radloff L.S. The CES-D Scale. A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [ Google Scholar ]
- Tarter R.E., Kirisci L., Mezzich A. Does the “gateway” sequence increase prediction of cannabis use disorder development beyond deviant socialization? Implications for prevention practice and policy. Drug Alcohol Depend. 2012;123S:S72–278. doi: 10.1016/j.drugalcdep.2012.01.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Trenz R., Scherer M., Harrell P., Zur J., Sinha A., Latimer W. Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addict. Behav. 2012;37:367–372. doi: 10.1016/j.addbeh.2011.11.011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang W., O'Brien N., Forrest J.I. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS ONE. 2012;7:e40793–e40801. doi: 10.1371/journal.pone.0040793. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (442.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Open access
- Published: 13 November 2021
Risk and protective factors of drug abuse among adolescents: a systematic review
- Azmawati Mohammed Nawi 1 ,
- Rozmi Ismail 2 ,
- Fauziah Ibrahim 2 ,
- Mohd Rohaizat Hassan 1 ,
- Mohd Rizal Abdul Manaf 1 ,
- Noh Amit 3 ,
- Norhayati Ibrahim 3 &
- Nurul Shafini Shafurdin 2
BMC Public Health volume 21 , Article number: 2088 ( 2021 ) Cite this article
159k Accesses
133 Citations
21 Altmetric
Metrics details
Drug abuse is detrimental, and excessive drug usage is a worldwide problem. Drug usage typically begins during adolescence. Factors for drug abuse include a variety of protective and risk factors. Hence, this systematic review aimed to determine the risk and protective factors of drug abuse among adolescents worldwide.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was adopted for the review which utilized three main journal databases, namely PubMed, EBSCOhost, and Web of Science. Tobacco addiction and alcohol abuse were excluded in this review. Retrieved citations were screened, and the data were extracted based on strict inclusion and exclusion criteria. Inclusion criteria include the article being full text, published from the year 2016 until 2020 and provided via open access resource or subscribed to by the institution. Quality assessment was done using Mixed Methods Appraisal Tools (MMAT) version 2018 to assess the methodological quality of the included studies. Given the heterogeneity of the included studies, a descriptive synthesis of the included studies was undertaken.
Out of 425 articles identified, 22 quantitative articles and one qualitative article were included in the final review. Both the risk and protective factors obtained were categorized into three main domains: individual, family, and community factors. The individual risk factors identified were traits of high impulsivity; rebelliousness; emotional regulation impairment, low religious, pain catastrophic, homework completeness, total screen time and alexithymia; the experience of maltreatment or a negative upbringing; having psychiatric disorders such as conduct problems and major depressive disorder; previous e-cigarette exposure; behavioral addiction; low-perceived risk; high-perceived drug accessibility; and high-attitude to use synthetic drugs. The familial risk factors were prenatal maternal smoking; poor maternal psychological control; low parental education; negligence; poor supervision; uncontrolled pocket money; and the presence of substance-using family members. One community risk factor reported was having peers who abuse drugs. The protective factors determined were individual traits of optimism; a high level of mindfulness; having social phobia; having strong beliefs against substance abuse; the desire to maintain one’s health; high paternal awareness of drug abuse; school connectedness; structured activity and having strong religious beliefs.
The outcomes of this review suggest a complex interaction between a multitude of factors influencing adolescent drug abuse. Therefore, successful adolescent drug abuse prevention programs will require extensive work at all levels of domains.
Peer Review reports
Introduction
Drug abuse is a global problem; 5.6% of the global population aged 15–64 years used drugs at least once during 2016 [ 1 ]. The usage of drugs among younger people has been shown to be higher than that among older people for most drugs. Drug abuse is also on the rise in many ASEAN (Association of Southeast Asian Nations) countries, especially among young males between 15 and 30 years of age. The increased burden due to drug abuse among adolescents and young adults was shown by the Global Burden of Disease (GBD) study in 2013 [ 2 ]. About 14% of the total health burden in young men is caused by alcohol and drug abuse. Younger people are also more likely to die from substance use disorders [ 3 ], and cannabis is the drug of choice among such users [ 4 ].
Adolescents are the group of people most prone to addiction [ 5 ]. The critical age of initiation of drug use begins during the adolescent period, and the maximum usage of drugs occurs among young people aged 18–25 years old [ 1 ]. During this period, adolescents have a strong inclination toward experimentation, curiosity, susceptibility to peer pressure, rebellion against authority, and poor self-worth, which makes such individuals vulnerable to drug abuse [ 2 ]. During adolescence, the basic development process generally involves changing relations between the individual and the multiple levels of the context within which the young person is accustomed. Variation in the substance and timing of these relations promotes diversity in adolescence and represents sources of risk or protective factors across this life period [ 6 ]. All these factors are crucial to helping young people develop their full potential and attain the best health in the transition to adulthood. Abusing drugs impairs the successful transition to adulthood by impairing the development of critical thinking and the learning of crucial cognitive skills [ 7 ]. Adolescents who abuse drugs are also reported to have higher rates of physical and mental illness and reduced overall health and well-being [ 8 ].
The absence of protective factors and the presence of risk factors predispose adolescents to drug abuse. Some of the risk factors are the presence of early mental and behavioral health problems, peer pressure, poorly equipped schools, poverty, poor parental supervision and relationships, a poor family structure, a lack of opportunities, isolation, gender, and accessibility to drugs [ 9 ]. The protective factors include high self-esteem, religiosity, grit, peer factors, self-control, parental monitoring, academic competence, anti-drug use policies, and strong neighborhood attachment [ 10 , 11 , 12 , 13 , 14 , 15 ].
The majority of previous systematic reviews done worldwide on drug usage focused on the mental, psychological, or social consequences of substance abuse [ 16 , 17 , 18 ], while some focused only on risk and protective factors for the non-medical use of prescription drugs among youths [ 19 ]. A few studies focused only on the risk factors of single drug usage among adolescents [ 20 ]. Therefore, the development of the current systematic review is based on the main research question: What is the current risk and protective factors among adolescent on the involvement with drug abuse? To the best of our knowledge, there is limited evidence from systematic reviews that explores the risk and protective factors among the adolescent population involved in drug abuse. Especially among developing countries, such as those in South East Asia, such research on the risk and protective factors for drug abuse is scarce. Furthermore, this review will shed light on the recent trends of risk and protective factors and provide insight into the main focus factors for prevention and control activities program. Additionally, this review will provide information on how these risk and protective factors change throughout various developmental stages. Therefore, the objective of this systematic review was to determine the risk and protective factors of drug abuse among adolescents worldwide. This paper thus fills in the gaps of previous studies and adds to the existing body of knowledge. In addition, this review may benefit certain parties in developing countries like Malaysia, where the national response to drugs is developing in terms of harm reduction, prison sentences, drug treatments, law enforcement responses, and civil society participation.
This systematic review was conducted using three databases, PubMed, EBSCOhost, and Web of Science, considering the easy access and wide coverage of reliable journals, focusing on the risk and protective factors of drug abuse among adolescents from 2016 until December 2020. The search was limited to the last 5 years to focus only on the most recent findings related to risk and protective factors. The search strategy employed was performed in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) checklist.
A preliminary search was conducted to identify appropriate keywords and determine whether this review was feasible. Subsequently, the related keywords were searched using online thesauruses, online dictionaries, and online encyclopedias. These keywords were verified and validated by an academic professor at the National University of Malaysia. The keywords used as shown in Table 1 .
Selection criteria
The systematic review process for searching the articles was carried out via the steps shown in Fig. 1 . Firstly, screening was done to remove duplicate articles from the selected search engines. A total of 240 articles were removed in this stage. Titles and abstracts were screened based on the relevancy of the titles to the inclusion and exclusion criteria and the objectives. The inclusion criteria were full text original articles, open access articles or articles subscribed to by the institution, observation and intervention study design and English language articles. The exclusion criteria in this search were (a) case study articles, (b) systematic and narrative review paper articles, (c) non-adolescent-based analyses, (d) non-English articles, and (e) articles focusing on smoking (nicotine) and alcohol-related issues only. A total of 130 articles were excluded after title and abstract screening, leaving 55 articles to be assessed for eligibility. The full text of each article was obtained, and each full article was checked thoroughly to determine if it would fulfil the inclusion criteria and objectives of this study. Each of the authors compared their list of potentially relevant articles and discussed their selections until a final agreement was obtained. A total of 22 articles were accepted to be included in this review. Most of the excluded articles were excluded because the population was not of the target age range—i.e., featuring subjects with an age > 18 years, a cohort born in 1965–1975, or undergraduate college students; the subject matter was not related to the study objective—i.e., assessing the effects on premature mortality, violent behavior, psychiatric illness, individual traits, and personality; type of article such as narrative review and neuropsychiatry review; and because of our inability to obtain the full article—e.g., forthcoming work in 2021. One qualitative article was added to explain the domain related to risk and the protective factors among the adolescents.
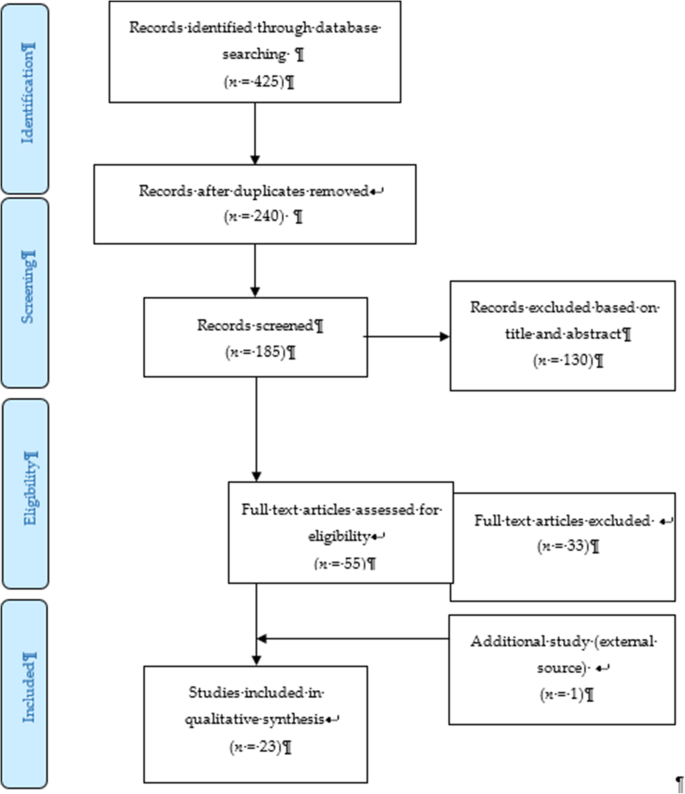
PRISMA flow diagram showing the selection of studies on risk and protective factors for drug abuse among adolescents.2.2. Operational Definition
Drug-related substances in this context refer to narcotics, opioids, psychoactive substances, amphetamines, cannabis, ecstasy, heroin, cocaine, hallucinogens, depressants, and stimulants. Drugs of abuse can be either off-label drugs or drugs that are medically prescribed. The two most commonly abused substances not included in this review are nicotine (tobacco) and alcohol. Accordingly, e-cigarettes and nicotine vape were also not included. Further, “adolescence” in this study refers to members of the population aged between 10 to 18 years [ 21 ].
Data extraction tool
All researchers independently extracted information for each article into an Excel spreadsheet. The data were then customized based on their (a) number; (b) year; (c) author and country; (d) titles; (e) study design; (f) type of substance abuse; (g) results—risks and protective factors; and (h) conclusions. A second reviewer crossed-checked the articles assigned to them and provided comments in the table.
Quality assessment tool
By using the Mixed Method Assessment Tool (MMAT version 2018), all articles were critically appraised for their quality by two independent reviewers. This tool has been shown to be useful in systematic reviews encompassing different study designs [ 22 ]. Articles were only selected if both reviewers agreed upon the articles’ quality. Any disagreement between the assigned reviewers was managed by employing a third independent reviewer. All included studies received a rating of “yes” for the questions in the respective domains of the MMAT checklists. Therefore, none of the articles were removed from this review due to poor quality. The Cohen’s kappa (agreement) between the two reviewers was 0.77, indicating moderate agreement [ 23 ].
The initial search found 425 studies for review, but after removing duplicates and applying the criteria listed above, we narrowed the pool to 22 articles, all of which are quantitative in their study design. The studies include three prospective cohort studies [ 24 , 25 , 26 ], one community trial [ 27 ], one case-control study [ 28 ], and nine cross-sectional studies [ 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. After careful discussion, all reviewer panels agreed to add one qualitative study [ 46 ] to help provide reasoning for the quantitative results. The selected qualitative paper was chosen because it discussed almost all domains on the risk and protective factors found in this review.
A summary of all 23 articles is listed in Table 2 . A majority of the studies (13 articles) were from the United States of America (USA) [ 25 , 26 , 27 , 29 , 30 , 31 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ], three studies were from the Asia region [ 32 , 33 , 38 ], four studies were from Europe [ 24 , 28 , 40 , 44 ], and one study was from Latin America [ 35 ], Africa [ 43 ] and Mediterranean [ 45 ]. The number of sample participants varied widely between the studies, ranging from 70 samples (minimum) to 700,178 samples (maximum), while the qualitative paper utilized a total of 100 interviewees. There were a wide range of drugs assessed in the quantitative articles, with marijuana being mentioned in 11 studies, cannabis in five studies, and opioid (six studies). There was also large heterogeneity in terms of the study design, type of drug abused, measurements of outcomes, and analysis techniques used. Therefore, the data were presented descriptively.
After thorough discussion and evaluation, all the findings (both risk and protective factors) from the review were categorized into three main domains: individual factors, family factors, and community factors. The conceptual framework is summarized in Fig. 2 .
Conceptual framework of risk and protective factors related to adolescent drug abuse

DOMAIN: individual factor
Risk factors.
Almost all the articles highlighted significant findings of individual risk factors for adolescent drug abuse. Therefore, our findings for this domain were further broken down into five more sub-domains consisting of personal/individual traits, significant negative growth exposure, personal psychiatric diagnosis, previous substance history, comorbidity and an individual’s attitude and perception.
Personal/individual traits
Chuang et al. [ 29 ] found that adolescents with high impulsivity traits had a significant positive association with drug addiction. This study also showed that the impulsivity trait alone was an independent risk factor that increased the odds between two to four times for using any drug compared to the non-impulsive group. Another longitudinal study by Guttmannova et al. showed that rebellious traits are positively associated with marijuana drug abuse [ 27 ]. The authors argued that measures of rebelliousness are a good proxy for a youth’s propensity to engage in risky behavior. Nevertheless, Wilson et al. [ 37 ], in a study involving 112 youths undergoing detoxification treatment for opioid abuse, found that a majority of the affected respondents had difficulty in regulating their emotions. The authors found that those with emotional regulation impairment traits became opioid dependent at an earlier age. Apart from that, a case-control study among outpatient youths found that adolescents involved in cannabis abuse had significant alexithymia traits compared to the control population [ 28 ]. Those adolescents scored high in the dimension of Difficulty in Identifying Emotion (DIF), which is one of the key definitions of diagnosing alexithymia. Overall, the adjusted Odds Ratio for DIF in cannabis abuse was 1.11 (95% CI, 1.03–1.20).
Significant negative growth exposure
A history of maltreatment in the past was also shown to have a positive association with adolescent drug abuse. A study found that a history of physical abuse in the past is associated with adolescent drug abuse through a Path Analysis, despite evidence being limited to the female gender [ 25 ]. However, evidence from another study focusing at foster care concluded that any type of maltreatment might result in a prevalence as high as 85.7% for the lifetime use of cannabis and as high as 31.7% for the prevalence of cannabis use within the last 3-months [ 30 ]. The study also found significant latent variables that accounted for drug abuse outcomes, which were chronic physical maltreatment (factor loading of 0.858) and chronic psychological maltreatment (factor loading of 0.825), with an r 2 of 73.6 and 68.1%, respectively. Another study shed light on those living in child welfare service (CWS) [ 35 ]. It was observed through longitudinal measurements that proportions of marijuana usage increased from 9 to 18% after 36 months in CWS. Hence, there is evidence of the possibility of a negative upbringing at such shelters.
Personal psychiatric diagnosis
The robust studies conducted in the USA have deduced that adolescents diagnosed with a conduct problem (CP) have a positive association with marijuana abuse (OR = 1.75 [1.56, 1.96], p < 0.0001). Furthermore, those with a diagnosis of Major Depressive Disorder (MDD) showed a significant positive association with marijuana abuse.
Previous substance and addiction history
Another study found that exposure to e-cigarettes within the past 30 days is related to an increase in the prevalence of marijuana use and prescription drug use by at least four times in the 8th and 10th grades and by at least three times in the 12th grade [ 34 ]. An association between other behavioral addictions and the development of drug abuse was also studied [ 29 ]. Using a 12-item index to assess potential addictive behaviors [ 39 ], significant associations between drug abuse and the groups with two behavioral addictions (OR = 3.19, 95% CI 1.25,9.77) and three behavioral addictions (OR = 3.46, 95% CI 1.25,9.58) were reported.
Comorbidity
The paper by Dash et al. (2020) highlight adolescent with a disease who needs routine medical pain treatment have higher risk of opioid misuse [ 38 ]. The adolescents who have disorder symptoms may have a risk for opioid misuse despite for the pain intensity.
Individual’s attitudes and perceptions
In a study conducted in three Latin America countries (Argentina, Chile, and Uruguay), it was shown that adolescents with low or no perceived risk of taking marijuana had a higher risk of abuse (OR = 8.22 times, 95% CI 7.56, 10.30) [ 35 ]. This finding is in line with another study that investigated 2002 adolescents and concluded that perceiving the drug as harmless was an independent risk factor that could prospectively predict future marijuana abuse [ 27 ]. Moreover, some youth interviewed perceived that they gained benefits from substance use [ 38 ]. The focus group discussion summarized that the youth felt positive personal motivation and could escape from a negative state by taking drugs. Apart from that, adolescents who had high-perceived availability of drugs in their neighborhoods were more likely to increase their usage of marijuana over time (OR = 11.00, 95% CI 9.11, 13.27) [ 35 ]. A cheap price of the substance and the availability of drug dealers around schools were factors for youth accessibility [ 38 ]. Perceived drug accessibility has also been linked with the authorities’ enforcement programs. The youth perception of a lax community enforcement of laws regarding drug use at all-time points predicted an increase in marijuana use in the subsequent assessment period [ 27 ]. Besides perception, a study examining the attitudes towards synthetic drugs based on 8076 probabilistic samples of Macau students found that the odds of the lifetime use of marijuana was almost three times higher among those with a strong attitude towards the use of synthetic drugs [ 32 ]. In addition, total screen time among the adolescent increase the likelihood of frequent cannabis use. Those who reported daily cannabis use have a mean of 12.56 h of total screen time, compared to a mean of 6.93 h among those who reported no cannabis use. Adolescent with more time on internet use, messaging, playing video games and watching TV/movies were significantly associated with more frequent cannabis use [ 44 ].
Protective factors
Individual traits.
Some individual traits have been determined to protect adolescents from developing drug abuse habits. A study by Marin et al. found that youth with an optimistic trait were less likely to become drug dependent [ 33 ]. In this study involving 1104 Iranian students, it was concluded that a higher optimism score (measured using the Children Attributional Style Questionnaire, CASQ) was a protective factor against illicit drug use (OR = 0.90, 95% CI: 0.85–0.95). Another study found that high levels of mindfulness, measured using the 25-item Child Acceptance and Mindfulness Measure, CAMM, lead to a slower progression toward injectable drug abuse among youth with opioid addiction (1.67 years, p = .041) [ 37 ]. In addition, the social phobia trait was found to have a negative association with marijuana use (OR = 0.87, 95% CI 0.77–0.97), as suggested [ 31 ].
According to El Kazdouh et al., individuals with a strong belief against substance use and those with a strong desire to maintain their health were more likely to be protected from involvement in drug abuse [ 46 ].
DOMAIN: family factors
The biological factors underlying drug abuse in adolescents have been reported in several studies. Epigenetic studies are considered important, as they can provide a good outline of the potential pre-natal factors that can be targeted at an earlier stage. Expecting mothers who smoke tobacco and alcohol have an indirect link with adolescent substance abuse in later life [ 24 , 39 ]. Moreover, the dynamic relationship between parents and their children may have some profound effects on the child’s growth. Luk et al. examined the mediator effects between parenting style and substance abuse and found the maternal psychological control dimension to be a significant variable [ 26 ]. The mother’s psychological control was two times higher in influencing her children to be involved in substance abuse compared to the other dimension. Conversely, an indirect risk factor towards youth drug abuse was elaborated in a study in which low parental educational level predicted a greater risk of future drug abuse by reducing the youth’s perception of harm [ 27 , 43 ]. Negligence from a parental perspective could also contribute to this problem. According to El Kazdouh et al. [ 46 ], a lack of parental supervision, uncontrolled pocket money spending among children, and the presence of substance-using family members were the most common negligence factors.
While the maternal factors above were shown to be risk factors, the opposite effect was seen when the paternal figure equipped himself with sufficient knowledge. A study found that fathers with good information and awareness were more likely to protect their adolescent children from drug abuse [ 26 ]. El Kazdouh et al. noted that support and advice could be some of the protective factors in this area [ 46 ].
DOMAIN: community factors
- Risk factor
A study in 2017 showed a positive association between adolescent drug abuse and peers who abuse drugs [ 32 , 39 ]. It was estimated that the odds of becoming a lifetime marijuana user was significantly increased by a factor of 2.5 ( p < 0.001) among peer groups who were taking synthetic drugs. This factor served as peer pressure for youth, who subconsciously had desire to be like the others [ 38 ]. The impact of availability and engagement in structured and unstructured activities also play a role in marijuana use. The findings from Spillane (2000) found that the availability of unstructured activities was associated with increased likelihood of marijuana use [ 42 ].
- Protective factor
Strong religious beliefs integrated into society serve as a crucial protective factor that can prevent adolescents from engaging in drug abuse [ 38 , 45 ]. In addition, the school connectedness and adult support also play a major contribution in the drug use [ 40 ].
The goal of this review was to identify and classify the risks and protective factors that lead adolescents to drug abuse across the three important domains of the individual, family, and community. No findings conflicted with each other, as each of them had their own arguments and justifications. The findings from our review showed that individual factors were the most commonly highlighted. These factors include individual traits, significant negative growth exposure, personal psychiatric diagnosis, previous substance and addiction history, and an individual’s attitude and perception as risk factors.
Within the individual factor domain, nine articles were found to contribute to the subdomain of personal/ individual traits [ 27 , 28 , 29 , 37 , 38 , 39 , 40 , 43 , 44 ]. Despite the heterogeneity of the study designs and the substances under investigation, all of the papers found statistically significant results for the possible risk factors of adolescent drug abuse. The traits of high impulsivity, rebelliousness, difficulty in regulating emotions, and alexithymia can be considered negative characteristic traits. These adolescents suffer from the inability to self-regulate their emotions, so they tend to externalize their behaviors as a way to avoid or suppress the negative feelings that they are experiencing [ 41 , 47 , 48 ]. On the other hand, engaging in such behaviors could plausibly provide a greater sense of positive emotions and make them feel good [ 49 ]. Apart from that, evidence from a neurophysiological point of view also suggests that the compulsive drive toward drug use is complemented by deficits in impulse control and decision making (impulsive trait) [ 50 ]. A person’s ability in self-control will seriously impaired with continuous drug use and will lead to the hallmark of addiction [ 51 ].
On the other hand, there are articles that reported some individual traits to be protective for adolescents from engaging in drug abuse. Youth with the optimistic trait, a high level of mindfulness, and social phobia were less likely to become drug dependent [ 31 , 33 , 37 ]. All of these articles used different psychometric instruments to classify each individual trait and were mutually exclusive. Therefore, each trait measured the chance of engaging in drug abuse on its own and did not reflect the chance at the end of the spectrum. These findings show that individual traits can be either protective or risk factors for the drugs used among adolescents. Therefore, any adolescent with negative personality traits should be monitored closely by providing health education, motivation, counselling, and emotional support since it can be concluded that negative personality traits are correlated with high risk behaviours such as drug abuse [ 52 ].
Our study also found that a history of maltreatment has a positive association with adolescent drug abuse. Those adolescents with episodes of maltreatment were considered to have negative growth exposure, as their childhoods were negatively affected by traumatic events. Some significant associations were found between maltreatment and adolescent drug abuse, although the former factor was limited to the female gender [ 25 , 30 , 36 ]. One possible reason for the contrasting results between genders is the different sample populations, which only covered child welfare centers [ 36 ] and foster care [ 30 ]. Regardless of the place, maltreatment can happen anywhere depending on the presence of the perpetrators. To date, evidence that concretely links maltreatment and substance abuse remains limited. However, a plausible explanation for this link could be the indirect effects of posttraumatic stress (i.e., a history of maltreatment) leading to substance use [ 53 , 54 ]. These findings highlight the importance of continuous monitoring and follow-ups with adolescents who have a history of maltreatment and who have ever attended a welfare center.
Addiction sometimes leads to another addiction, as described by the findings of several studies [ 29 , 34 ]. An initial study focused on the effects of e-cigarettes in the development of other substance abuse disorders, particularly those related to marijuana, alcohol, and commonly prescribed medications [ 34 ]. The authors found that the use of e-cigarettes can lead to more severe substance addiction [ 55 ], possibly through normalization of the behavior. On the other hand, Chuang et al.’s extensive study in 2017 analyzed the combined effects of either multiple addictions alone or a combination of multiple addictions together with the impulsivity trait [ 29 ]. The outcomes reported were intriguing and provide the opportunity for targeted intervention. The synergistic effects of impulsiveness and three other substance addictions (marijuana, tobacco, and alcohol) substantially increased the likelihood for drug abuse from 3.46 (95%CI 1.25, 9.58) to 10.13 (95% CI 3.95, 25.95). Therefore, proper rehabilitation is an important strategy to ensure that one addiction will not lead to another addiction.
The likelihood for drug abuse increases as the population perceives little or no harmful risks associated with the drugs. On the opposite side of the coin, a greater perceived risk remains a protective factor for marijuana abuse [ 56 ]. However, another study noted that a stronger determinant for adolescent drug abuse was the perceived availability of the drug [ 35 , 57 ]. Looking at the bigger picture, both perceptions corroborate each other and may inform drug use. Another study, on the other hand, reported that there was a decreasing trend of perceived drug risk in conjunction with the increasing usage of drugs [ 58 ]. As more people do drugs, youth may inevitably perceive those drugs as an acceptable norm without any harmful consequences [ 59 ].
In addition, the total spent for screen time also contribute to drug abuse among adolescent [ 43 ]. This scenario has been proven by many researchers on the effect of screen time on the mental health [ 60 ] that leads to the substance use among the adolescent due to the ubiquity of pro-substance use content on the internet. Adolescent with comorbidity who needs medical pain management by opioids also tend to misuse in future. A qualitative exploration on the perspectives among general practitioners concerning the risk of opioid misuse in people with pain, showed pain management by opioids is a default treatment and misuse is not a main problem for the them [ 61 ]. A careful decision on the use of opioids as a pain management should be consider among the adolescents and their understanding is needed.
Within the family factor domain, family structures were found to have both positive and negative associations with drug abuse among adolescents. As described in one study, paternal knowledge was consistently found to be a protective factor against substance abuse [ 26 ]. With sufficient knowledge, the father can serve as the guardian of his family to monitor and protect his children from negative influences [ 62 ]. The work by Luk et al. also reported a positive association of maternal psychological association towards drug abuse (IRR 2.41, p < 0.05) [ 26 ]. The authors also observed the same effect of paternal psychological control, although it was statistically insignificant. This construct relates to parenting style, and the authors argued that parenting style might have a profound effect on the outcomes under study. While an earlier literature review [ 63 ] also reported such a relationship, a recent study showed a lesser impact [ 64 ] with regards to neglectful parenting styles leading to poorer substance abuse outcomes. Nevertheless, it was highlighted in another study that the adolescents’ perception of a neglectful parenting style increased their odds (OR 2.14, p = 0.012) of developing alcohol abuse, not the parenting style itself [ 65 ]. Altogether, families play vital roles in adolescents’ risk for engaging in substance abuse [ 66 ]. Therefore, any intervention to impede the initiation of substance use or curb existing substance use among adolescents needs to include parents—especially improving parent–child communication and ensuring that parents monitor their children’s activities.
Finally, the community also contributes to drug abuse among adolescents. As shown by Li et al. [ 32 ] and El Kazdouh et al. [ 46 ], peers exert a certain influence on other teenagers by making them subconsciously want to fit into the group. Peer selection and peer socialization processes might explain why peer pressure serves as a risk factor for drug-abuse among adolescents [ 67 ]. Another study reported that strong religious beliefs integrated into society play a crucial role in preventing adolescents from engaging in drug abuse [ 46 ]. Most religions devalue any actions that can cause harmful health effects, such as substance abuse [ 68 ]. Hence, spiritual beliefs may help protect adolescents. This theme has been well established in many studies [ 60 , 69 , 70 , 71 , 72 ] and, therefore, could be implemented by religious societies as part of interventions to curb the issue of adolescent drug abuse. The connection with school and structured activity did reduce the risk as a study in USA found exposure to media anti-drug messages had an indirect negative effect on substances abuse through school-related activity and social activity [ 73 ]. The school activity should highlight on the importance of developmental perspective when designing and offering school-based prevention programs [75].
Limitations
We adopted a review approach that synthesized existing evidence on the risk and protective factors of adolescents engaging in drug abuse. Although this systematic review builds on the conclusion of a rigorous review of studies in different settings, there are some potential limitations to this work. We may have missed some other important factors, as we only included English articles, and article extraction was only done from the three search engines mentioned. Nonetheless, this review focused on worldwide drug abuse studies, rather than the broader context of substance abuse including alcohol and cigarettes, thereby making this paper more focused.
Conclusions
This review has addressed some recent knowledge related to the individual, familial, and community risk and preventive factors for adolescent drug use. We suggest that more attention should be given to individual factors since most findings were discussed in relation to such factors. With the increasing trend of drug abuse, it will be critical to focus research specifically on this area. Localized studies, especially those related to demographic factors, may be more effective in generating results that are specific to particular areas and thus may be more useful in generating and assessing local control and prevention efforts. Interventions using different theory-based psychotherapies and a recognition of the unique developmental milestones specific to adolescents are among examples that can be used. Relevant holistic approaches should be strengthened not only by relevant government agencies but also by the private sector and non-governmental organizations by promoting protective factors while reducing risk factors in programs involving adolescents from primary school up to adulthood to prevent and control drug abuse. Finally, legal legislation and enforcement against drug abuse should be engaged with regularly as part of our commitment to combat this public health burden.
Data availability and materials
All data generated or analysed during this study are included in this published article.
Nation, U. World Drug Report 2018 (United Nations publication, Sales No. E.18X.XI.9. United Nation publication). 2018. Retrieved from https://www.unodc.org/wdr2018
Google Scholar
Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry. 2016;3(3):251–64. https://doi.org/10.1016/S2215-0366(15)00508-8 Elsevier Ltd.
Article PubMed Google Scholar
Ritchie H, Roser M. Drug Use - Our World in Data: Global Change Data Lab; 2019. https://ourworldindata.org/drug-use [10 June 2020]
Holm S, Sandberg S, Kolind T, Hesse M. The importance of cannabis culture in young adult cannabis use. J Subst Abus. 2014;19(3):251–6.
Luikinga SJ, Kim JH, Perry CJ. Developmental perspectives on methamphetamine abuse: exploring adolescent vulnerabilities on brain and behavior. Progress Neuro Psychopharmacol Biol Psychiatry. 2018;87(Pt A):78–84. https://doi.org/10.1016/j.pnpbp.2017.11.010 Elsevier Inc.
Article CAS Google Scholar
Ismail R, Ghazalli MN, Ibrahim N. Not all developmental assets can predict negative mental health outcomes of disadvantaged youth: a case of suburban Kuala Lumpur. Mediterr J Soc Sci. 2015;6(1):452–9. https://doi.org/10.5901/mjss.2015.v6n5s1p452 .
Article Google Scholar
Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–99. https://doi.org/10.1016/j.pbb.2006.12.001 .
Article CAS PubMed Google Scholar
Schulte MT, Hser YI. Substance use and associated health conditions throughout the lifespan. Public Health Rev. 2013;35(2). https://doi.org/10.1007/bf03391702 Technosdar Ltd.
Somani, S.; Meghani S. Substance Abuse among Youth: A Harsh Reality 2016. doi: https://doi.org/10.4172/2165-7548.1000330 , 6, 4.
Book Google Scholar
Drabble L, Trocki KF, Klinger JL. Religiosity as a protective factor for hazardous drinking and drug use among sexual minority and heterosexual women: findings from the National Alcohol Survey. Drug Alcohol Depend. 2016;161:127–34. https://doi.org/10.1016/j.drugalcdep.2016.01.022 .
Article PubMed PubMed Central Google Scholar
Goliath V, Pretorius B. Peer risk and protective factors in adolescence: Implications for drug use prevention. Soc Work. 2016;52(1):113–29. https://doi.org/10.15270/52-1-482 .
Guerrero LR, Dudovitz R, Chung PJ, Dosanjh KK, Wong MD. Grit: a potential protective factor against substance use and other risk behaviors among Latino adolescents. Acad Pediatr. 2016;16(3):275–81. https://doi.org/10.1016/j.acap.2015.12.016 .
National Institutes on Drug Abuse. What are risk factors and protective factors? National Institute on Drug Abuse (NIDA); 2003. Retrieved from https://www.drugabuse.gov/publications/preventing-drug-use-among-children-adolescents/chapter-1-risk-factors-protective-factors/what-are-risk-factors
Nguyen NN, Newhill CE. The role of religiosity as a protective factor against marijuana use among African American, White, Asian, and Hispanic adolescents. J Subst Abus. 2016;21(5):547–52. https://doi.org/10.3109/14659891.2015.1093558 .
Schinke S, Schwinn T, Hopkins J, Wahlstrom L. Drug abuse risk and protective factors among Hispanic adolescents. Prev Med Rep. 2016;3:185–8. https://doi.org/10.1016/j.pmedr.2016.01.012 .
Macleod J, Oakes R, Copello A, Crome PI, Egger PM, Hickman M, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363(9421):1579–88. https://doi.org/10.1016/S0140-6736(04)16200-4 .
Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–28. https://doi.org/10.1016/S0140-6736(07)61162-3 .
Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187–94. https://doi.org/10.1177/0269881105049040 .
Nargiso JE, Ballard EL, Skeer MR. A systematic review of risk and protective factors associated with nonmedical use of prescription drugs among youth in the united states: A social ecological perspective. J Stud Alcohol Drugs. 2015;76(1):5–20. https://doi.org/10.15288/jsad.2015.76.5 .
Guxensa M, Nebot M, Ariza C, Ochoa D. Factors associated with the onset of cannabis use: a systematic review of cohort studies. Gac Sanit. 2007;21(3):252–60. https://doi.org/10.1157/13106811 .
Susan MS, Peter SA, Dakshitha W, George CP. The age of adolescence. Lancet Child Adolesc Health. 2018;2(Issue 3):223–8. https://doi.org/10.1016/S2352-4642(18)30022-1 .
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285–91. https://doi.org/10.3233/EFI-180221 .
McHugh ML. Interrater reliability: The kappa statistic. Biochem Med. 2012;22(3):276–82. https://doi.org/10.11613/bm.2012.031 .
Cecil CAM, Walton E, Smith RG, Viding E, McCrory EJ, Relton CL, et al. DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Transl Psychiatry. 2016;6(12):e976. https://doi.org/10.1038/tp.2016.247 Nature Publishing Group.
Article CAS PubMed PubMed Central Google Scholar
Kobulsky JM. Gender differences in pathways from physical and sexual abuse to early substance use. Child Youth Serv Rev. 2017;83:25–32. https://doi.org/10.1016/j.childyouth.2017.10.027 .
Luk JW, King KM, McCarty CA, McCauley E, Stoep A. Prospective effects of parenting on substance use and problems across Asian/Pacific islander and European American youth: Tests of moderated mediation. J Stud Alcohol Drugs. 2017;78(4):521–30. https://doi.org/10.15288/jsad.2017.78.521 .
Guttmannova K, Skinner ML, Oesterle S, White HR, Catalano RF, Hawkins JD. The interplay between marijuana-specific risk factors and marijuana use over the course of adolescence. Prev Sci. 2019;20(2):235–45. https://doi.org/10.1007/s11121-018-0882-9 .
Dorard G, Bungener C, Phan O, Edel Y, Corcos M, Berthoz S. Is alexithymia related to cannabis use disorder? Results from a case-control study in outpatient adolescent cannabis abusers. J Psychosom Res. 2017;95:74–80. https://doi.org/10.1016/j.jpsychores.2017.02.012 .
Chuang CWI, Sussman S, Stone MD, Pang RD, Chou CP, Leventhal AM, et al. Impulsivity and history of behavioral addictions are associated with drug use in adolescents. Addict Behav. 2017;74:41–7. https://doi.org/10.1016/j.addbeh.2017.05.021 .
Gabrielli J, Jackson Y, Brown S. Associations between maltreatment history and severity of substance use behavior in youth in Foster Care. Child Maltreat. 2016;21(4):298–307. https://doi.org/10.1177/1077559516669443 .
Khoddam R, Jackson NJ, Leventhal AM. Internalizing symptoms and conduct problems: redundant, incremental, or interactive risk factors for adolescent substance use during the first year of high school? Drug Alcohol Depend. 2016;169:48–55. https://doi.org/10.1016/j.drugalcdep.2016.10.007 .
Li SD, Zhang X, Tang W, Xia Y. Predictors and implications of synthetic drug use among adolescents in the gambling Capital of China. SAGE Open. 2017;7(4):215824401773303. https://doi.org/10.1177/2158244017733031 .
Marin S, Heshmatian E, Nadrian H, Fakhari A, Mohammadpoorasl A. Associations between optimism, tobacco smoking and substance abuse among Iranian high school students. Health Promot Perspect. 2019;9(4):279–84. https://doi.org/10.15171/hpp.2019.38 .
Miech RA, O’Malley PM, Johnston LD, Patrick ME. E-cigarettes and the drug use patterns of adolescents. Nicotine Tob Res. 2015;18(5):654–9. https://doi.org/10.1093/ntr/ntv217 .
Schleimer JP, Rivera-Aguirre AE, Castillo-Carniglia A, Laqueur HS, Rudolph KE, Suárez H, et al. Investigating how perceived risk and availability of marijuana relate to marijuana use among adolescents in Argentina, Chile, and Uruguay over time. Drug Alcohol Depend. 2019;201:115–26. https://doi.org/10.1016/j.drugalcdep.2019.03.029 .
Traube DE, Yarnell LM, Schrager SM. Differences in polysubstance use among youth in the child welfare system: toward a better understanding of the highest-risk teens. Child Abuse Negl. 2016;52:146–57. https://doi.org/10.1016/j.chiabu.2015.11.020 .
Wilson JD, Vo H, Matson P, Adger H, Barnett G, Fishman M. Trait mindfulness and progression to injection use in youth with opioid addiction. Subst Use Misuse. 2017;52(11):1486–93. https://doi.org/10.1080/10826084.2017.1289225 .
Dash GF, Feldstein Ewing SW, Murphy C, Hudson KA, Wilson AC. Contextual risk among adolescents receiving opioid prescriptions for acute pain in pediatric ambulatory care settings. Addict Behav. 2020;104:106314. https://doi.org/10.1016/j.addbeh.2020.106314 Epub 2020 Jan 11. PMID: 31962289; PMCID: PMC7024039.
Osborne V, Serdarevic M, Striley CW, Nixon SJ, Winterstein AG, Cottler LB. Age of first use of prescription opioids and prescription opioid non-medical use among older adolescents. Substance Use Misuse. 2020;55(14):2420–7. https://doi.org/10.1080/10826084.2020.1823420 .
Zuckermann AME, Qian W, Battista K, Jiang Y, de Groh M, Leatherdale ST. Factors influencing the non-medical use of prescription opioids among youth: results from the COMPASS study. J Subst Abus. 2020;25(5):507–14. https://doi.org/10.1080/14659891.2020.1736669 .
De Pedro KT, Esqueda MC, Gilreath TD. School protective factors and substance use among lesbian, gay, and bisexual adolescents in California public schools. LGBT Health. 2017;4(3):210–6. https://doi.org/10.1089/lgbt.2016.0132 .
Spillane NS, Schick MR, Kirk-Provencher KT, Hill DC, Wyatt J, Jackson KM. Structured and unstructured activities and alcohol and marijuana use in middle school: the role of availability and engagement. Substance Use Misuse. 2020;55(11):1765–73. https://doi.org/10.1080/10826084.2020.1762652 .
Ogunsola OO, Fatusi AO. Risk and protective factors for adolescent substance use: a comparative study of secondary school students in rural and urban areas of Osun state, Nigeria. Int J Adolesc Med Health. 2016;29(3). https://doi.org/10.1515/ijamh-2015-0096 .
Doggett A, Qian W, Godin K, De Groh M, Leatherdale ST. Examining the association between exposure to various screen time sedentary behaviours and cannabis use among youth in the COMPASS study. SSM Population Health. 2019;9:100487. https://doi.org/10.1016/j.ssmph.2019.100487 .
Afifi RA, El Asmar K, Bteddini D, Assi M, Yassin N, Bitar S, et al. Bullying victimization and use of substances in high school: does religiosity moderate the association? J Relig Health. 2020;59(1):334–50. https://doi.org/10.1007/s10943-019-00789-8 .
El Kazdouh H, El-Ammari A, Bouftini S, El Fakir S, El Achhab Y. Adolescents, parents and teachers’ perceptions of risk and protective factors of substance use in Moroccan adolescents: a qualitative study. Substance Abuse Treat Prevent Policy. 2018;13(1):–31. https://doi.org/10.1186/s13011-018-0169-y .
Sussman S, Lisha N, Griffiths M. Prevalence of the addictions: a problem of the majority or the minority? Eval Health Prof. 2011;34(1):3–56. https://doi.org/10.1177/0163278710380124 .
Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30(2):217–37. https://doi.org/10.1016/j.cpr.2009.11.004 .
Ricketts T, Macaskill A. Gambling as emotion management: developing a grounded theory of problem gambling. Addict Res Theory. 2003;11(6):383–400. https://doi.org/10.1080/1606635031000062074 .
Williams AD, Grisham JR. Impulsivity, emotion regulation, and mindful attentional focus in compulsive buying. Cogn Ther Res. 2012;36(5):451–7. https://doi.org/10.1007/s10608-011-9384-9 .
National Institutes on Drug Abuse. Drugs, brains, and behavior the science of addiction national institute on drug abuse (nida). 2014. Retrieved from https://www.drugabuse.gov/sites/default/files/soa_2014.pdf
Hokm Abadi ME, Bakhti M, Nazemi M, Sedighi S, Mirzadeh Toroghi E. The relationship between personality traits and drug type among substance abuse. J Res Health. 2018;8(6):531–40.
Longman-Mills S, Haye W, Hamilton H, Brands B, Wright MGM, Cumsille F, et al. Psychological maltreatment and its relationship with substance abuse among university students in Kingston, Jamaica, vol. 24. Florianopolis: Texto Contexto Enferm; 2015. p. 63–8.
Rosenkranz SE, Muller RT, Henderson JL. The role of complex PTSD in mediating childhood maltreatment and substance abuse severity among youth seeking substance abuse treatment. Psychol Trauma Theory Res Pract Policy. 2014;6(1):25–33. https://doi.org/10.1037/a0031920 .
Krishnan-Sarin S, Morean M, Kong G, et al. E-Cigarettes and “dripping” among high-school youth. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-3224 .
Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard review of psychiatry. NIH Public Access. 2004;12(6):305–20. https://doi.org/10.1080/10673220490910844 .
Kandel D, Kandel E. The gateway hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatrica. 2014;104(2):130–7.
Dempsey RC, McAlaney J, Helmer SM, Pischke CR, Akvardar Y, Bewick BM, et al. Normative perceptions of Cannabis use among European University students: associations of perceived peer use and peer attitudes with personal use and attitudes. J Stud Alcohol Drugs. 2016;77(5):740–8.
Cioffredi L, Kamon J, Turner W. Effects of depression, anxiety and screen use on adolescent substance use. Prevent Med Rep. 2021;22:101362. https://doi.org/10.1016/j.pmedr.2021.101362 .
Luckett T, NewtonJohn T, Phillips J, et al. Risk of opioid misuse in people with cancer and pain and related clinical considerations:a qualitative study of the perspectives of Australian general practitioners. BMJ Open. 2020;10(2):e034363. https://doi.org/10.1136/bmjopen-2019-034363 .
Lipari RN. Trends in Adolescent Substance Use and Perception of Risk from Substance Use. The CBHSQ Report. Substance Abuse Mental Health Serv Admin. 2013; Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27656743 .
Muchiri BW, dos Santos MML. Family management risk and protective factors for adolescent substance use in South Africa. Substance Abuse. 2018;13(1):24. https://doi.org/10.1186/s13011-018-0163-4 .
Becoña E, Martínez Ú, Calafat A, Juan M, Fernández-Hermida JR, Secades-Villa R. Parental styles and drug use: a review. In: Drugs: Education, Prevention and Policy: Taylor & Francis; 2012. https://doi.org/10.3109/09687637.2011.631060 .
Berge J, Sundel K, Ojehagen A, Hakansson A. Role of parenting styles in adolescent substance use: results from a Swedish longitudinal cohort study. BMJ Open. 2016;6(1):e008979. https://doi.org/10.1136/bmjopen-2015-008979 .
Opara I, Lardier DT, Reid RJ, Garcia-Reid P. “It all starts with the parents”: a qualitative study on protective factors for drug-use prevention among black and Hispanic girls. Affilia J Women Soc Work. 2019;34(2):199–218. https://doi.org/10.1177/0886109918822543 .
Martínez-Loredo V, Fernández-Artamendi S, Weidberg S, Pericot I, López-Núñez C, Fernández-Hermida J, et al. Parenting styles and alcohol use among adolescents: a longitudinal study. Eur J Invest Health Psychol Educ. 2016;6(1):27–36. https://doi.org/10.1989/ejihpe.v6i1.146 .
Baharudin MN, Mohamad M, Karim F. Drug-abuse inmates maqasid shariah quality of lifw: a conceotual paper. Hum Soc Sci Rev. 2020;8(3):1285–94. https://doi.org/10.18510/hssr.2020.83131 .
Henneberger AK, Mushonga DR, Preston AM. Peer influence and adolescent substance use: a systematic review of dynamic social network research. Adolesc Res Rev. 2020;6(1):57–73. https://doi.org/10.1007/s40894-019-00130-0 Springer.
Gomes FC, de Andrade AG, Izbicki R, Almeida AM, de Oliveira LG. Religion as a protective factor against drug use among Brazilian university students: a national survey. Rev Bras Psiquiatr. 2013;35(1):29–37. https://doi.org/10.1016/j.rbp.2012.05.010 .
Kulis S, Hodge DR, Ayers SL, Brown EF, Marsiglia FF. Spirituality and religion: intertwined protective factors for substance use among urban American Indian youth. Am J Drug Alcohol Abuse. 2012;38(5):444–9. https://doi.org/10.3109/00952990.2012.670338 .
Miller L, Davies M, Greenwald S. Religiosity and substance use and abuse among adolescents in the national comorbidity survey. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1190–7. https://doi.org/10.1097/00004583-200009000-00020 .
Moon SS, Rao U. Social activity, school-related activity, and anti-substance use media messages on adolescent tobacco and alcohol use. J Hum Behav Soc Environ. 2011;21(5):475–89. https://doi.org/10.1080/10911359.2011.566456 .
Simone A. Onrust, Roy Otten, Jeroen Lammers, Filip smit, school-based programmes to reduce and prevent substance use in different age groups: what works for whom? Systematic review and meta-regression analysis. Clin Psychol Rev. 2016;44:45–59. https://doi.org/10.1016/j.cpr.2015.11.002 .
Download references
Acknowledgements
The authors acknowledge The Ministry of Higher Education Malaysia and The Universiti Kebangsaan Malaysia, (UKM) for funding this study under the Long-Term Research Grant Scheme-(LGRS/1/2019/UKM-UKM/2/1). We also thank the team for their commitment and tireless efforts in ensuring that manuscript was well executed.
Financial support for this study was obtained from the Ministry of Higher Education, Malaysia through the Long-Term Research Grant Scheme-(LGRS/1/2019/UKM-UKM/2/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Department of Community Health, Universiti Kebangsaan Malaysia, Cheras, 56000, Kuala Lumpur, Malaysia
Azmawati Mohammed Nawi, Mohd Rohaizat Hassan & Mohd Rizal Abdul Manaf
Centre for Research in Psychology and Human Well-Being (PSiTra), Faculty of Social Sciences and Humanities, Universiti Kebangsaan Malaysia, 43600, Bangi, Selangor, Malaysia
Rozmi Ismail, Fauziah Ibrahim & Nurul Shafini Shafurdin
Clinical Psychology and Behavioural Health Program, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Noh Amit & Norhayati Ibrahim
You can also search for this author in PubMed Google Scholar
Contributions
Manuscript concept, and drafting AMN and RI; model development, FI, NI and NA.; Editing manuscript MRH, MRAN, NSS,; Critical revision of manuscript for important intellectual content, all authors. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Rozmi Ismail .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of the Secretariat of Research Ethics, Universiti Kebangsaan Malaysia, Faculty of Medicine, Cheras, Kuala Lumpur (Reference no. UKMPPI/111/8/JEP-2020.174(2). Dated 27 Mac 2020.
Consent for publication
Not applicable.
Competing interests
The authors AMN, RI, FI, MRM, MRAM, NA, NI NSS declare that they have no conflict of interest relevant to this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nawi, A.M., Ismail, R., Ibrahim, F. et al. Risk and protective factors of drug abuse among adolescents: a systematic review. BMC Public Health 21 , 2088 (2021). https://doi.org/10.1186/s12889-021-11906-2
Download citation
Received : 10 June 2021
Accepted : 22 September 2021
Published : 13 November 2021
DOI : https://doi.org/10.1186/s12889-021-11906-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drug abuse, substance, adolescent
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
National Institute on Drug Abuse Division of Research 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office ... be encouraged to generate future research aimed at hypothesis and theory testing. Marvin Snyder, Ph.D. Director, Division of Research National Institute on Drug Abuse xi.
151. Research Pap er. Theoretical Fr amework on Theories and Effectiveness of Substance Abuse. 1 Vimala Periyannanpillai and *2 Dr. KSS Rakesh. 1 PhD Scholar, IIC University of Technology ...
Examining the Gateway Hypothesis. This book represents the first systematic discussion of the Gateway Hypothesis, a developmental hypothesis formulated to model how adolescents initiate and progress in the use of various drugs. In the United States, this progression pro-ceeds from the use of tobacco or alcohol to the use of marijuana and other ...
The Gateway Hypothesis describes how tobacco or alcohol use precedes marijuana and other illicit drug use. We review the epidemiological data, explore the underlying molecular mechanisms in mice and discuss the societal implications of the hypothesis, including the use of e-cigarettes by young people. Conclusion
Abstract. Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially exploitable therapeutically. In particular, basic studies have documented a reduction in the electrophysiological activity of DA neurons in alcohol, opiate, cannabinoid ...
effects of long-term drug abuse throughout the body. For example, research has shown that tobacco smoke causes cancer of the mouth, throat, larynx, blood, lungs, stomach, pancreas, kidney, bladder, and cervix.19 In addition, some drugs of abuse, such as inhalants, are toxic to nerve cells and may damage or destroy them.
Consistent with the theory, research in the substance abuse literature has focused on age of onset of substance used as a proximate determinant of future drug use and dependence in adulthood (Chen et al., 2009, Behrendt et al., 2009, Trenz et al., 2012, Mayet et al., 2012). Trenz et al. (2012) found early onset of alcohol at age 15 but not ...
As Becker (1963) notes: Marihuana-produced sensations are not automatically or necessarily pleasurable. The taste for such an experience is a socially acquired one, not different from acquired tastes for oysters or dry martinis. The user feels dizzy, thirsty; his scalp tingles; he misjudges time and distances.
The gateway drug hypothesis refers to the pattern of substance use during adolescence whereby legal substances, such as nicotine and alcohol, precede the progressive use of illicit substances like ...
Abstract and Figures. Dopamine (DA) transmission is deeply affected by drugs of abuse, and alterations in DA function are involved in the various phases of drug addiction and potentially ...
Substance or Drug abuse is a serious public health problem affecting usually adolescents and young adults. It affects both males and females and it is. the major source of crimes in youth and ...
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
1: Understanding Drug Abuse and Addi. tion: What Science Says2: Drug addiction: a complex illnessDr. g addiction is a complex illness. The path to drug addiction begins with. he act of taking drugs. Over time, a person's ability to choose not to take drugs is compromised. This, in large. art, is a result of the effects of prolonged drug use on ...
Principles of Drug Addiction Treatment: A Research-Based Guide (Third Edition) Published in 2014, this report offered health professionals and other stakeholders information on principles of effective drug addiction treatment, answers to frequently asked questions, an overview of the drug addiction treatment landscape in the United States, and ...
Background Drug abuse is detrimental, and excessive drug usage is a worldwide problem. Drug usage typically begins during adolescence. Factors for drug abuse include a variety of protective and risk factors. Hence, this systematic review aimed to determine the risk and protective factors of drug abuse among adolescents worldwide. Methods Preferred Reporting Items for Systematic Reviews and ...
44. Drugs, delinquency and crime are related in many ways. In some cases, drug abuse may lead to crime; in others, criminal behaviour precedes drug abuse. The broader impact of drug abuse and crime may increase tension and other deviance, placing additional burdens on institutions such as the family.
The Relationship Between Crime. and Drugs: What W e Ha ve. Learned in Recent Decades. David Deitch, Ph.D.*; Igor Koutsenok M.D.** & Amanda Ruiz, M.D.***. Abstract —The focus of this article is ...
b) Effects of Drug Abuse: The signs or harmful effects of drug abuse could be physical, emotional, family d ynamics, school behaviours, a nd social problems. They include cardio vascular. disease ...
mental illness with suspicions, intemperate fears, mood disorders and. depressi on. Narcotics and liquor damage the liver, stomach, brain and. nerves which results memory loss, restlessness and so ...
PDF | This paper viewed youth as a vulnerable population that is susceptible to drug addiction and abuse in the society. In order words, youth's use of... | Find, read and cite all the research ...