Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- JME Commentaries
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 47, Issue 2
- Good reasons to vaccinate: mandatory or payment for risk?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1691-6403 Julian Savulescu 1 , 2 , 3
- 1 Faculty of Philosophy , University of Oxford , Oxford , UK
- 2 Murdoch Childrens Research Institute , Parkville , Victoria , Australia
- 3 Melbourne Law School , University of Melbourne , Melbourne , Victoria , Australia
- Correspondence to Professor Julian Savulescu, Faculty of Philosophy, University of Oxford, Oxford, UK; julian.savulescu{at}philosophy.ox.ac.uk
Mandatory vaccination, including for COVID-19, can be ethically justified if the threat to public health is grave, the confidence in safety and effectiveness is high, the expected utility of mandatory vaccination is greater than the alternatives, and the penalties or costs for non-compliance are proportionate. I describe an algorithm for justified mandatory vaccination. Penalties or costs could include withholding of benefits, imposition of fines, provision of community service or loss of freedoms. I argue that under conditions of risk or perceived risk of a novel vaccination, a system of payment for risk in vaccination may be superior. I defend a payment model against various objections, including that it constitutes coercion and undermines solidarity. I argue that payment can be in cash or in kind, and opportunity for altruistic vaccinations can be preserved by offering people who have been vaccinated the opportunity to donate any cash payment back to the health service.
- behaviour modification
- technology/risk assessment
- philosophical ethics
- public health ethics
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/medethics-2020-106821
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
We are in the midst of a global pandemic with COVID-19 and there is a race to develop a vaccine. At the time of writing, there are 53 vaccines in clinical trials on humans (plus five that have bypassed the full trial process) and at least 92 preclinical vaccines under active investigation in animals. There are a number of different approaches: (1) genetic—using mRNA to cause the body to produce viral proteins; (2) viral vector—using genetically modified viruses such as adenovirus to carry sections of coronavirus genetic material; (3) protein—delivering viral proteins (but not genetic material) to provoke an immune response; (4) inactivated or attenuated coronavirus; (5) repurposing existing vaccines, eg, BCG (bacillus Calmette–Guérin). 1
Given the mounting number of deaths globally, and the apparent failure of many countries to contain the pandemic without severely damaging or problematic lockdowns and other measures, there have been calls to make a vaccine, if it were approved, mandatory. 2 Mandatory vaccination has not been ruled out within the UK. 3
The first part of this article asks when, if ever, a vaccine should be mandatory. I will create a set of criteria and a decision algorithm for mandatory vaccination. I will argue that in the case of COVID-19, some of these criteria may not be satisfied. The second part of the article argues that in the case of COVID-19, it may be ethically preferable to incentivise vaccine uptake. I will justify incentivisation and discuss different kinds of incentives.
Ethics of mandatory COVID-19 vaccination
There is a large body of literature on the justification for the use of coercion in public health and infectious disease in particular. Mandatory vaccination is typically justified on Millian grounds: harm to others. According to John Stuart Mill, the sole ground for the use of state coercion (and restriction of liberty) is when one individual risks harming others. 4 The most prominent arguments from bioethicists appeal to preventing harm to others. 5–7 In the case of children, significant risk of harm to the child is also a ground for state protection. Bambery et al 8 give the example of a child taking a box of toxic bleach to school, potentially harming himself and other children. Teachers are entitled to restrain the child and remove the poison both because of risk to the child and to other children. 8 Flanigan uses a similar example of a person shooting a gun into a crowd. 5
The Nuffield Council of Bioethics produced an influential report on public health which considers when coercion and mandatory vaccination might be justified:
When assessing whether more directive policies are acceptable, the following factors should be taken into account: the risks associated with the vaccination and with the disease itself, and the seriousness of the threat of the disease to the population. In the case of incentivised policies, the size of the incentive involved should be appropriate so that it would not unduly compromise the voluntariness of consent. We identified two circumstances in which quasi-mandatory vaccination measures are more likely to be justified. First, for highly contagious and serious diseases, for example with characteristics similar to smallpox. Second, for disease eradication if the disease is serious and if eradication is within reach. 9
I will elaborate on these brief suggestions and provide a novel structured algorithm for when vaccination should be mandatory.
COVID-19 is almost unique because of the gravity of the problem at the global level: not only is there cost in terms of lives from COVID-19, there is also the extraordinary economic, health and well-being consequences of various virus-control measures, including lockdown, which will extend into the future. Probably never before has a vaccine been developed so rapidly and the pressure to use it so great, at least at the global level.
There is a strong case for making any vaccination mandatory (or compulsory) if four conditions are met:
There is a grave threat to public health
The vaccine is safe and effective
Mandatory vaccination has a superior cost/benefit profile compared with other alternatives
The level of coercion is proportionate.
Each of these conditions involves value judgements.
Grave threat to public health
So far, there have been over 1 million deaths attributed to COVID-19 globally (as of 30 September 2020). 10 In the UK alone, it was predicted in influential early modelling that 500 000 would have died if nothing was done to prevent its spread. Even with the subsequent introduction of a range of highly restrictive lockdown measures (measures which could themselves come at a cost of 200 000 non-COVID-19 lives according to a recent UK government report), 11 more than 42 000 (as of 30 September 2020) 12 have died in the UK within 28 days of a positive test.
The case fatality rate was originally estimated to be as high as 11%, but (as is typical with new diseases) this was quickly scaled down to 1.5% or even lower. 13 The infection fatality rate (IFR, which accounts for asymptomatic and undiagnosed cases) is lower still as it has become clear that there are a large number of asymptomatic and mild cases. For example, the Centre for Evidence Based Medicine reports that “In Iceland, where the most testing per capita has occurred, the IFR lies somewhere between 0.03% and 0.28%”. 14
Of course, how you define “grave” is a value judgement. One of the worst-affected countries in the world in terms of COVID-19-attributed deaths per million is Belgium. The mortality is (at the time of writing) around 877 per million population, which is still under 0.1%, and the average age of death is 80. Of course, Belgium and most other countries have taken strict measures to control the virus and so we are not seeing the greatest possible impact the virus could have. Yet others such as Brazil and Sweden have intervened to a much lesser degree, yet (currently) have rates of 687 and 578 deaths per million respectively. Sweden’s April all-cause deaths and death rate at the peak of its pandemic so far, while extremely high, were surpassed by months in 1993 and 2000. 15
The data are complex and difficult to compare with different testing rates, and ways of assigning deaths and collecting data differing from country to country. For example, Belgium counts deaths in care homes where there is a suspicion that COVID-19 was the cause (without the need for a positive test) and, until recently, the UK counted a death which followed any time from a COVID-19 positive test as a COVID-19 death. Moreover, there have been huge behavioural changes even in countries without legally enforced lockdowns. Furthermore, the IFR varies wildly by age-group and other factors. Even among survivors, there is emerging evidence that there may be long-term consequences for those who have been infected but survived. Long COVID-19 health implications may present a grave future public health problem. Nevertheless, some might still argue that this disease has not entered the “grave” range that would warrant mandatory vaccination. The Spanish influenza killed many more (50–100 million), 16 and it afflicted younger rather than older people, meaning even more “life years” were lost. It is not difficult to imagine a Superflu, or bioengineered bug, which killed 10% across all ages. This would certainly be a grave public health emergency where it is likely mandatory vaccination would be employed.
Deciding whether COVID-19 is sufficiently grave requires both more data than we have and also a value judgement over the gravity that would warrant this kind of intervention. But let us grant for the sake of argument that COVID-19 is a grave public health emergency.
Vaccine is safe and effective
There are concerns that testing has been rushed and the vaccine may not be safe or effective. 17
First, although the technology being used in many of these vaccine candidates has been successfully used in other vaccines, no country has ever produced a safe and effective vaccine against a coronavirus. So in one way, we are all in uncharted waters.
Second, any vaccine development will be accelerated in the context of a grave public health emergency.The inherent probabilistic nature of the development of any biologic means that no vaccine could be said to be 100% safe. There will be risks and those risks are likely to be greater than with well-established vaccines.
Thirdly, some side effects may take time to manifest.
This is not to support the anti-vaccination movement. Vaccines are one of the greatest medical accomplishments and a cornerstone of public health. There are robust testing procedures in place in most jurisdictions to ensure that licensed COVID-19 vaccines are both effective and safe. It is only to acknowledge that everything, including vaccination, has risks. Perhaps the biggest challenge in the development of a vaccine for COVID-19 will be to be honest about the extent of those risks and convey the limitations of confidence in safety and efficacy relative to the evidence accrued.
There is an ethical balance to be struck: introducing a vaccine early and saving more lives from COVID-19, but risking side effects or ineffectiveness versus engaging in longer and more rigorous testing, and having more confidence in safety and efficacy, but more people dying of COVID-19 while such testing occurs. There is no magic answer and, given the economic, social and health catastrophe of various anti-COVID-19 measures such as lockdown, there will be considerable pressure to introduce a vaccine earlier.
To be maximally effective, particularly in protecting the most vulnerable in the population, vaccination would need to achieve herd immunity (the exact percentage of the population that would need to be immune for herd immunity to be reached depends on various factors, but current estimates range up to 82% of the population). 18
There are huge logistical issues around finding a vaccine, proving it to be safe, and then producing and administering it to the world’s population. Even if those issues are resolved, the pandemic has come at a time where there is another growing problem in public health: vaccine hesitancy.
US polls “suggest only 3 in 4 people would get vaccinated if a COVID-19 vaccine were available, and only 30% would want to receive the vaccine soon after it becomes available.” 18
Indeed, vaccine refusal appears to be going up. A recent Pew survey suggested 49% of adults in the USA would refuse a COVID-19 vaccine in September 2020. 19
If these results prove accurate then even if a safe and effective vaccine is produced, at best, herd immunity will be significantly delayed by vaccine hesitancy at a cost both to lives and to the resumption of normal life, and at worst, it may never be achieved.
There remain some community concerns about the safety of all pre-existing vaccines, including many that have been rigorously tested and employed for years.
In the case of COVID-19, the hesitancy may be exacerbated by the accelerated testing and approval process which applies not only to Sputnik V (the controversial “Russian vaccine”). Speaking about America’s vaccine programme, Warp Speed, Donald Trump applauded its unprecedented pace:
…my administration cut through every piece of red tape to achieve the fastest-ever, by far, launch of a vaccine trial for this new virus, this very vicious virus. And I want to thank all of the doctors and scientists and researchers involved because they’ve never moved like this, or never even close. 20
The large impact on society means the vaccine will be put to market much more quickly than usual, perhaps employing challenge studies or other innovative designs, or by condensing or running certain non-safety critical parts of the process in parallel (for example, creating candidate vaccines before its approval).
While the speed is welcomed by politicians and some members of the public, the pressure to produce a candidate vaccine, and the speed at which it has been done, may be also perceived (perhaps unfairly) to increase the likelihood of the kind of concerns that lead to vaccine hesitancy: concerns over side-effects that are unexpected or rare, or that take longer to appear than the testing process allows for, or that for another reason may be missed in the testing process.
The job to be done will not only be to prove scientifically that the vaccine is safe and effective, but also to inform and reassure the public, especially the group who are willing to take the vaccine in theory—but only after others have tried it out first.
The question remains of how safe is safe enough to warrant mandatory vaccination. It is vanishingly unlikely that there will be absolutely no risk of harm from any biomedical intervention, and the disease itself has dramatically different risk profiles in different groups of the population. In an ideal world, the vaccine would be proven to be 100% safe. But there will likely be some risk remaining. Any mandatory vaccination programme would therefore need to make a value judgement about what level of safety and what level of certainty are safe and certain enough. Of course, it would need to be very high, but a 0% risk option is very unlikely.
A COVID-19 vaccine may be effective in reducing community spread and/or preventing disease in individuals. Mandatory vaccination is most justifiable when there are benefits to both the individual and in terms of preventing transmission. If the benefits are only to individual adults, it is more difficult to support mandatory vaccination. One justification would be to prevent exhaustion of healthcare services in an emergency (eg, running out of ventilators), which has been used a basis of restriction of liberty (it was the main justification for lockdown). It could also be justified in the case of protection of children and others who cannot decide for themselves, and of other adults who either cannot be vaccinated for medical reasons.
Better than the alternatives
It is a standard principle of decision theory that the expected utility of a proposed option must be compared with the expected utility of relevant alternatives. There are many alternatives to mandatory vaccination. See figure 1 for a summary of the range of strategies for preventing infectious disease.
- Download figure
- Open in new tab
- Download powerpoint
Strategies for prevention of infectious disease.
A popular position, especially among medical professionals, 7 is that we don’t need mandatory vaccination because people are self-interested or altruistic enough to come forward for vaccination. We can reach herd immunity without mandatory vaccination.
If this were true, all well and good, but the surveys mentioned above cast doubt on this claim with regard to the future COVID-19 vaccine. Moreover, reaching herd immunity is not good enough.
First, how fast we reach herd immunity is also important. In a pandemic, time is lives. If it takes a year to reach herd immunity, that could be thousands or tens of thousands of lives in one country.
Second, herd immunity is necessary because some people cannot be vaccinated for medical reasons: they have allergies, immune problems, or other illnesses. The elderly often don’t mount a strong immune response (that is why it is better to vaccinate children for influenza because they are the biggest spreaders of that disease 7 —although COVID-19 appears to be different on the current evidence). And immunity wanes over time—so even people previously vaccinated may become vulnerable.
Even when national herd immunity is achieved, local areas can fall below that level over time, causing outbreaks, as happened with measles recently. This is especially likely to happen where people opposed to vaccines tend to cluster toghether—for example, in the case of certain religious communities. So ideally we need better than herd immunity to ensure that people are protected both over time and in every place.
These are thus reasons to doubt whether a policy of voluntary vaccination will be good enough, though it remains to be seen.
There are other policies that might obviate the need for mandatory vaccination. South Korea has kept deaths down to about 300 (at the time of writing) with a population of 60 000 000 with a vigorous track and trace programme (although it was criticised for exposing extra-marital affairs and other stigmatised behaviours). 21 Other countries have enforced quarantine with tracking devices. There could be selective lockdown of certain groups, 22 or for intermittent periods of time.
The long-term costs and benefits of such policies would have to be evaluated. That is, we should calculate the expected utility of mandatory vaccination (in combination with other policies) and compare it to alternative strategies (or some other combination of these). How utility should be evaluated is an ethical question. Should we count deaths averted (no matter how old), life years lost or lost well-being (perhaps measured by quality adjusted life years)? 23 Should we count loss of liberty or privacy into the other side the equation?
It may be that a one-off mandatory vaccination is a significantly smaller loss of well-being or liberty than these other complex resource intensive strategies.
So we cannot say whether a mandatory policy of COVID-19 vaccination is ethically justified until we can assess the nature of the vaccine, the gravity of the problem and the likely costs/benefit of alternatives. But it is certainly feasible that it could be justified.
It is important to recognise that coercive vaccination can be justified. This is easy to see by comparing it to other coercive interventions in the public interest.
Conscription in war
In the gravest emergencies, where the existence and freedom of the whole population is at stake, people are conscripted to serve their country, often with high risk of death or permanent injury. We often analogise the pandemic to a war: we are fighting the virus. If people can be sent to war against their will, in certain circumstances some levels of coercion are justified in the war on the virus. Notably, in conditions of extreme emergency in past wars (graver than currently exist for COVID-19), imprisonment or compulsion have even been employed. 24
A more mundane example is the payment of taxes. Taxes benefit individuals because tax revenue allows the preservation of public goods. But if sufficient numbers of others are paying their taxes, it is in a person’s self-interest to free ride and avoid taxes. Indeed, paying taxes may result in harm in some circumstances. 24 In the USA, where there is a large private healthcare sector, paying your taxes may mean you cannot pay for lifesaving medical care that you would otherwise have been able to afford. Still, taxes are mandatory based on considerations of fairness and utility.
Seat belts are mandatory in the UK and many other countries, whereas they were previously voluntary. Interestingly, 50% or so of Americans initially opposed making seat belts mandatory, but now 70% believe mandatory laws are justified. 25
Seat belts reduce the chance of death if you are involved in a car accident by 50%. They are very safe and effective. Notably, they do cause injuries (seat belt syndrome) and even, very occasionally, death. But the chances of being benefitted by wearing them vastly outweigh these risks, so they are mandatory, with enforcement through fines . I have previously likened vaccination to wearing a seat belt. 25
Pre-existing mandatory vaccination
Mandatory vaccination policies are already in place in different parts of the world. Mandatory vaccination policies are those that include a non-voluntary element to vaccine consent and impose a penalty or cost for unjustified refusal (justified refusal includes those who have a contraindicating medical condition, or those who already have natural immunity). There are a range of possible penalties or costs which can coerce people. Australia has the “No Jab, No Pay” scheme which withholds child benefits if the child is not vaccinated, and a “No Jab, No Play” scheme which withholds kindergarten childcare benefits. Italy introduced fines for unvaccinated children who attend school. In the USA, state regulations mandate that children cannot attend school if they are not vaccinated, and healthcare workers are required to vaccinate. Some US states (eg, Michigan) make exemptions difficult to obtain by requiring parents to attend immunisation education courses 26 (see also 27 28 ).
Figure 2 summarises the range of coercive policies that can constitute mandatory vaccination.
Cost of mandatory/coercive vaccination.
Coercion is proportionate
In public health ethics, there is a familiar concept of the “least restrictive alternative”. 28 The least restrictive alternative is the option which achieves a given outcome with the least coercion (and least restriction of liberty).
This is a very weak principle: it uses liberty as tie breaker between options with the same expected utility. More commonly, however, we need to weigh utility against liberty. That is, a more restrictive policy will achieve more expected utility—but is it justified?
According to a principle of proportionality, the additional coercion or infringement in liberty is justified if it is proportionate to the gain in expected utility of the more coercive intervention compared with next best option. That is, additional coercion is justified when the restriction of liberty is both minimised and proportionate to the expected advantages offered by the more coercive policy.
As we can see from the previous section and figure 2, there are a variety of coercive measures. (The Nuffield Council has created a related “Intervention Ladder”, 29 though this includes education and incentives, as well as coercive measures.) Penalties can be high. In war, those who conscientiously objected to fighting went to jail or were forced to perform community service (or participate in medical research). In France, parents were given a suspended prison sentence for refusing to vaccinate their child. 30
While there are legitimate concerns that the effectiveness of these policies in different contexts has been inadequately investigated, a number of these policies have been shown to increase vaccination rates. 31
Notably, the fine or punishment for avoiding taxes varies according to the gravity of the offence. The fine for not wearing a seat belt is typically small. A modest penalty for not being vaccinated in a grave public health emergency could be justifiable. For example, a fine or restriction of movement might be justified.
Figure 3 combines these four factors into an algorithm for justified mandatory vaccination.
Algorithm for mandatory vaccination.
These four factors can be justified in several ways. They represent a distillation and development of existing principles in public health ethics, for example, the least restrictive alternative. They can also be justified by the four principles of biomedical ethics.
For example, justice is about the distribution of benefits and burdens across a population in a fair manner. Justice and beneficence, in the context of vaccination policies, both require that the problem addressed is significant and vaccination is an effective means of addressing it. Non-maleficence requires that the risk imposed on individuals be small. Respect for autonomy and justice both require that coercion be applied only if necessary and that it be proportionate to additional utility of mandatory vaccination (and that such coercion be minimised, which is a feature of proportionality).
It is important to recognise that vaccines may have benefits both to the individual and to others (the community). If the vaccine has an overall net expected utility for the individual, beneficence supports its administration.
How great a sacrifice (loss of liberty or risk) can be justified? The most plausible account is provided by a duty of easy rescue: when the cost to an individual is small of some act, but the benefit or harm to another is large, then there is a moral obligation to perform that act. I have elsewhere argued for a collective duty of easy rescue: where the cost of some act to an individual is small, and the benefit of everyone doing that act to the collective is large, there is a collective duty of easy rescue. 32 Such a principle appropriately balances respect for autonomy with justice.
Whether mandatory vaccination for any disease can be justified will depend on precise facts around the magnitude of the problem, the nature of the disease and vaccination, the availability and effectiveness of alternative strategies and the level of coercion. Elsewhere I compare mandatory vaccination for influenza and COVID-19 in more detail. 27
Better than coercion? Payment for risk
Given the risks, or perceived risks, of a novel COVID-19 vaccine, it would be practically and perhaps ethically problematic to introduce a mandatory policy, at least initially (when uncertainty around safety will be greater). Is there a more attractive alternative?
The arguments in favour of vaccination, particularly for those at lower risk (children, young people and those previously infected) can be based on a principle of solidarity. After all, “We are in this together” has been a recurrent slogan supporting pandemic measures in different countries. Those at low risk are asked to do their duty to their fellow citizens, which is a kind of community service. Yet they have little to personally gain from vaccination. The risk/benefit profile looms large for those at lowest risk.
However, another way of looking at this is that those at low risk are being asked to do a job which entails some risk., so they should be paid for the risk they are taking for the sake of providing a public good. And although it may be unlikely to influence so-called 'anti-vaxxers', it may influence a good portion of the 60% of American adults who responded in a March 2020 poll that they would either delay vaccination or didn’t know about vaccination. 33
I have previously argued that we should reconceive live organ donation and participation in risky research, including challenge studies, 34 as jobs where risk should be remunerated, much like we pay construction workers and other dangerous professions both for the job and for the risk involved. 35 36 While the risk profile for approved vaccinations means that it differs from these examples, it could be compared to a job such as social work as a further argument in favour of payment. People may legitimately be incentivised to take on risks, as the Nuffield Council recognises in its Intervention Ladder. 29
The advantage of payment for risk is that people are choosing voluntarily to take it on. As long as we are accurate in conveying the limitations in our confidence about the risks and benefits of a vaccine, then it is up to individuals to judge whether they are worth payment.
Of course, that is a big ask. It would require government to be transparent, explicit and comprehensive in disclosure of data, what should be inferred and the limitations on the data and confidence. This has often not been the case—one only has to remember the denial of the risks of bovine spongiform encephalopathy (BSE) at the height of the crisis by the British government, when in 1990 the Minister for Agriculture, Fisheries and Food, John Gummer proudly fed his 4-year-old daughter, Cordelia, a hamburger in front of the world’s media, declaring British beef safe. (Gummer was awarded a peerage in 2010 and is now Lord Deben.) 37
There is also a danger that payment might signal lack of confidence in safety. That is a real risk and one that I will address in the “payment in kind” section below.
But the basic ethical point (public acceptability aside) is that, if a vaccine is judged to be safe enough to be used without payment, then it is safe enough to be used with payment. 36 Payment itself does not make a vaccine riskier. If a vaccine is considered too risky to be administered to the population, then it should not be administered, no matter whether coercively, through incentives, or through some other policy.
A standard objection to payment for risk (whether it is risky research or live organ donation) is that it is coercive: it forces people to take risks against their better judgement. In Macklin’s words:
The reason for holding that it is ethically inappropriate to pay patients to be research subjects is that it is likely to be coercive, violating the ethical requirement that participation in research should be fully voluntary. 38
As I have previously argued, 39 this demonstrates deep conceptual confusion. Coercion exists when an option which is either desired or good is removed or made very unappealing. The standard example is a robber who demands “Your money or your life”. This removes the most desired and best option: your money and your life. The Australian “No Jab, No Pay”scheme arguably does constitute coercion as it removes an option that one is entitled to, that is, non-vaccination with the “Pay”. So too is the Italian scheme of fines coercive.
However, paying people is not coercive. Adding an option, like payment, without affecting the status quo is not coercive. If a person chooses that option, it is because they believe that overall their life will go better with it, in this case, with the vaccination and the payment. The gamble may not pay off: some risk might eventuate and then it wasn’t worth it. But that is life—and probability.
It is true that the value of the option might exercise force over our rational capacities, but that is no different from offering a lot of money to attract a favoured job applicant.
What can be problematic about offers is exploitation. Exploitation exists where one offers less than a fair deal and a person only accepts it because of vulnerability from background injustice.
There are two ways to prevent exploitation. First, we can correct any background injustice that might cause it. In this case, the person would have little reason to accept the offer. Second, we can pay a fair minimum price for risk, as when we pay construction workers danger money. Paradoxically, this requires paying more, rather than less. 40
But there is an important additional feature of vaccination. If a vaccine were deemed to be safe enough to offer on a voluntary basis without payment, it must be safe enough to incentivise with payment because the risks are reasonable. It may be that those who are poorer may be more inclined to take the money and the risk, but this applies to all risky or unpleasant jobs in a market economy. It is not necessarily exploitation if there are protections in place such as a minimum wage or a fair price is paid to take on risk.
So payment for vaccination which passes independent safety standards (and could reasonably be offered without payment) is not exploitation, if the payment is adequate.
Undue influence?
A related concern is undue influence. Undue influence means that because of the attractiveness of the offer, I can’t autonomously and rationally weigh up the risks and benefits. It is sometimes understood as “were it not for the money, he would not do it”.
But that formulation is too broad—were it not for the money, many people would not go to work. Rather what the concept of ‘undue influence’ intends to capture is that the offer, usually money, bedazzles a person so that he or she makes a mistake in weighing up the risks and benefits. Someone offers Jones a million dollars to take on a risk of 99.99% of dying in a dangerous experiment. He just focuses on the money and takes a deal which is unfair and unreasonable. However, taking such an offer might be rational. If Jones’ daughter is about to die without a million dollars and he values her life more than his own, it might be both autonomous and rational to take the deal.
Because we cannot get into people’s minds, it is difficult in practice to unravel whether undue influence is occurring—how can you differentiate it from a rational decision? In practice, if it would be acceptable to be vaccinated for nothing, it is acceptable to do it for money. Concerns about undue influence are best met by implementing procedures to minimise bias and irrational decision making, such as cooling off periods, information reframing, and so on.
There remains a lurking concern that a decision where payment is involved may not be fully autonomous or authentic. For example, racial and ethnic minorities are among the groups most gravely affected by COVID-19, but given concerns about systemic racism in research and medicine, these communities may have good reason to distrust the medical machine. Is it acceptable to use payment to get over those concerns?
All we can do practically to address concerns about autonomy and authenticity is to redouble efforts: to ensure we do know the risks and they are reasonable (and that the underpinning research is not itself subject to concerns about systemic racism), and try to foster trust with such public education campaigns. This can work alongside a payment scheme. People still need to understand what the facts are. They still need to make as autonomous and authentic a decision as possible.
Practical advantages
A payment model could also be superior to a mandatory model from a practical point of view. There may be considerable resistance to a mandatory model which may make it difficult, expensive and time-consuming to implement, with considerable invasion of liberty. In a payment model, people are doing what they want to do.
A payment model could also be very cheap, compared with the alternatives. The cost of the UK’s furlough scheme is estimated to reach £60 billion by its planned end in October, 41 and the economic shut down is likely to cost many billions more, as well as the estimated 200 000 lives expected to be lost as a result. 11 It would make economic sense to pay people quite a lot to incentivise them to vaccinate sooner rather than later—which, for example, would speed up their full return to work.
It may be that payment is only required to incentivise certain groups. For example, it may be that young people require incentivising because they are at lower risk from the disease itself. On the other hand, justice might require payment for all taking the risk. Although the elderly and those at higher risk have more to gain personally, they are also providing a service by being vaccinated and not using limited health resources. (There is an enormous backlog of patients in the NHS—another grave threat to public health.)
One particularly difficult case is paying parents to vaccinate their children. It is one thing to pay people to take on risk for themselves; it is quite another to pay them to enable their children to take on risks, particularly when the children have little to gain as they are at lowest risk. In part, the answer to this issue is determined by how safe the vaccine is and how confident we can be in that assessment. If it were safe, to a level that even a mandatory programme would be justified, it may be appropriate to instead incentivise parents to volunteer their children for vaccination. If safety is less certain, payment for risk in this group is the most problematic.
It is true that some mandatory vaccination programmes already fine parents for failure to vaccinate their children. However, in those cases vaccination is clearly in the child’s best interest, as the child receives the benefit of immunity to diseases such as measles, that pose a greater risk to that child than we currently believe COVID-19 does. Moreover, they are for vaccines that have been in place for many years and have a well-established safety profile.
A standard objection to paying people to do their duty, particularly civic duty, is that it undermines solidarity, trust, reciprocity and other community values. This is the argument given by Richard Titmuss for a voluntary blood donation scheme. 42
The UK does not pay donors for blood or blood products, but does purchase blood products from other countries, including Austria where donors are paid a “travel allowance” for plasma donation. In Australia, which runs a donor system, more than 50% of the plasma comes from paid donors in the USA. 43 Altruism is insufficient. Germany recently moved to paying for plasma donors. It does not appear to have undermined German society.
In the end, the policy we should adopt towards COVID-19 vaccination will depend on the precise risks and benefits of the vaccine (and our confidence in them), the state of the pandemic, the nature of the alternatives, and particularly the public appetite for a vaccine.
In the right circumstances, mandatory vaccination could be ethically justified, if the penalty is suitably proportionate.
Payment for vaccination, perhaps, has even more to be said for it.
For those attached to the gift of altruism, the vaccinated could be offered the opportunity to donate their fee back to the NHS (or similar health service provider). This combined “payment-donation” model would be a happy marriage of ethics and economics. It would give altruists a double chance to be altruistic: first by vaccinating and second by donating the fee. It would also couple self-interest with morality for free-riders (as they would have greater self-interest to do what is moral), and it would give those who face obstacles to vaccination an additional reason to overcome these.
Payment in kind
Of course, benefits can come in cash or kind. An alternative “payment” model is to pay those who vaccinate in kind. This could take the form of greater freedom to travel, opportunity to work or socialise. With some colleagues, I have given similar arguments in favour of immunity passports. 44
One attractive benefit would be the freedom to not wear a mask in public places if you carried a vaccination certificate, and not to socially distance. Currently, everyone has to wear a mask and practise social distancing. Relaxing this requirement for those who have been vaccinated (or otherwise have immunity) would be an attractive benefit. Moreover, it would help ameliorate the risks the unvaccinated would pose to others.
Payment in kind has one advantage over cash in that it might not send the signal that vaccination is perceived to be unsafe. A cash payment may paradoxically undermine vaccination uptake by introducing unwarranted suspicion (though this is an intuition that may need to be tested). Benefits in kind are less susceptible to this concern because they are directly linked to the benefit provided by the vaccine itself: the vaccinated person is no longer a threat to others.
Some might object that this represents a form of shaming the non-vaccinators (some of whom might be excluded from vaccination for health reasons), just as presenting those who evaded conscription with a white feather was a method of shaming perceived free-riders. But this could be managed through an education campaign about the justification for face covering requirements. There is a good reason to require the non-vaccinated to continue to wear masks and practice social distancing, regardless of whether their refusal is justified—they do represent a greater direct threat to others.
It is quite possible that some mixture of altruism, financial and non-financial benefits will obviate the need to introduce mandatory vaccination. It is better that people voluntarily choose on the basis of reasons to act well, rather than being forced to do so. Structuring the rewards and punishments in a just and fair way is one way of giving people reasons for action.
Mandatory vaccination can be ethically justified (see figure 3), but when risks are more uncertain, payment for vaccination (whether in cash or kind) may be an ethically superior option.
Acknowledgments
This piece builds on a previous piece I published on the JME blog, Good Reasons to Vaccinate: COVID19 Vaccine, Mandatory or Payment Model? [ https://blogs.bmj.com/medical-ethics/2020/07/29/good-reasons-to-vaccinate-covid19-vaccine-mandatory-or-payment-model/ ]. I would like to thank an anonymous reviewer for very many helpful and constructive comments. I would also like to thank Alberto Giubilini for his help.
- Zimmer C , et al
- Bambery B ,
- Douglas T ,
- Selgelid MJ , et al
- Selgelid M ,
- Maslen H , et al
- Nuffield Council on Bioethics
- Worldometer
- ↵ Gov.uk. coronavirus (COVID-19) in the UK . Available: https://coronavirus.data.gov.uk/ [Accessed 30 Sep 2020 ].
- Rajgor DD ,
- Archuleta S , et al
- Johnson S ,
- Greenberger M
- Schaffer DeRoo S ,
- Pudalov NJ ,
- Johnson C ,
- Whitehouse.gov
- Savulescu J ,
- Persson I ,
- Wilkinson D
- Giubilini A
- Giubilini A ,
- Savulescu J
- Sabin Vaccine Institute
- MacDonald NE ,
- Dube E , et al
- LX Morning Consult
- Weijer C , et al
- Anomaly J ,
- Grimwade O ,
- Giubilini A , et al
- Brown RCH ,
- Williams B , et al
Supplementary materials
- Press release
Contributors Sole authorship.
Funding JS is supported by the Uehiro Foundation on Ethics and Education. He received funding from the Wellcome Trust WT104848 and WT203132. Through his involvement with the Murdoch Children’s Research Institute, he has received funding through from the Victorian State Government through the Operational Infrastructure Support (OIS) Program.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available.
Linked Articles
- Response Persuasion, not coercion or incentivisation, is the best means of promoting COVID-19 vaccination Susan Pennings Xavier Symons Journal of Medical Ethics 2021; 47 709-711 Published Online First: 27 Jan 2021. doi: 10.1136/medethics-2020-107076
Read the full text or download the PDF:
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
A comprehensive analysis of the efficacy and safety of COVID-19 vaccines
Changjing cai, yinghui peng, edward shen, qiaoqiao huang, yihong chen, ziyang feng, xiangyang zhang.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Shan Zeng, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China. [email protected]
Corresponding author: Hong Shen, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China. [email protected]
Corresponding author: Ying Han, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA. [email protected]
These authors contributed equally
Received 2021 May 12; Accepted 2021 Jul 31; Issue date 2021 Sep 1.
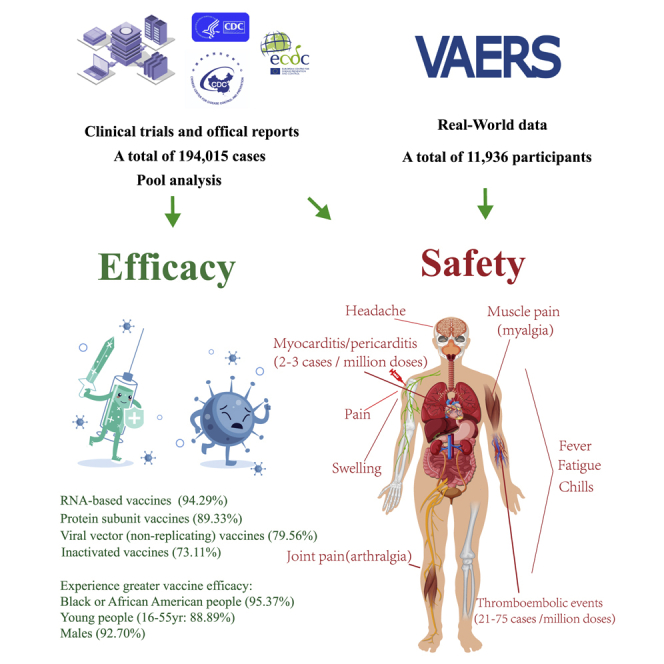
The numbers of cases and deaths from coronavirus disease 2019 (COVID-19) are continuously increasing. Many people are concerned about the efficacy and safety of the COVID-19 vaccines. We performed a comprehensive analysis of the published trials of COVID-19 vaccines and the real-world data from the Vaccine Adverse Event Reporting System. Globally, our research found that the efficacy of all vaccines exceeded 70%, and RNA-based vaccines had the highest efficacy of 94.29%; moreover, Black or African American people, young people, and males may experience greater vaccine efficacy. The spectrum of vaccine-related adverse drug reactions (ADRs) is extremely broad, and the most frequent ADRs are pain, fatigue, and headache. Most ADRs are tolerable and are mainly grade 1 or 2 in severity. Some severe ADRs have been identified (thromboembolic events, 21–75 cases per million doses; myocarditis/pericarditis, 2–3 cases per million doses). In summary, vaccines are a powerful tool that can be used to control the COVID-19 pandemic, with high efficacy and tolerable ADRs. In addition, the spectrum of ADRs associated with the vaccines is broad, and most of the reactions appear within a week, although some may be delayed. Therefore, ADRs after vaccination need to be identified and addressed in a timely manner.
Keywords: COVID-19, SARS-CoV-2, vaccine, safety, efficacy
Graphical abstract
The numbers of cases and deaths from COVID-19 are continuously increasing. Cai et al. are the first to comprehensively analyze the efficacy of the existing COVID-19 vaccines and the incidence, spectrum, timing, and clinical features of adverse reactions associated with the COVID-19 vaccines, which can provide reference for general public.
Introduction
As of April 5, 2021, there were more than 131 million confirmed cases and more than 2.8 million deaths due to coronavirus disease 2019 (COVID-19) worldwide. 1 COVID-19 has posed a serious threat to public health worldwide. There is no cure for COVID-19, and only vaccines can stop the spread of the COVID-19 pandemic. According to the World Health Organization (WHO), as of April 5, 2021, 184 vaccines were being evaluated in the preclinical development stage, 85 were in the clinical evaluation stage, and some had partially passed through phase III clinical trials. 2 Vaccination against COVID-19 has now started in 161 locations, covering 91% of the global population. 3 However, the vaccination rates are still low; as of April 5, 2021, the highest rate of full vaccination was 56.2% in Israel, while those in other countries were all lower than 20%, and those in some countries were 0%. 4 A previous study pointed out that 53%–84% of the population needs to be vaccinated against COVID-19 to achieve herd immunity. 5 However, as various mutations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported, herd immunity is becoming more and more unrealistic, unless a vaccine to protect against different variants of SARS-CoV-2 can be developed. Other than protection, vaccination can reduce the severity of COVID-19 infection and be life saving. One of the key reasons for the low vaccination rate is that many people are concerned about the safety and efficacy of the COVID-19 vaccines.
However, no reports have addressed this issue satisfactorily. It is important to perform an analysis of the safety and efficacy of the COVID-19 vaccines. Therefore, we performed a comprehensive analysis to determine the incidence, spectrum, timing, and clinical features of adverse drug reactions (ADRs) and the efficacy of the COVID-19 vaccines.
First, we performed a meta-analysis of the published trials of the COVID-19 vaccines. Furthermore, we retrospectively obtained real-world data from the Vaccine Adverse Event Reporting System (VAERS), which is comanaged by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) of the United States of America. 6 In our research, we provided a complete overview of the COVID-19 vaccines in terms of the incidence, spectrum, timing, and clinical features of ADRs and efficacy. We do hope this study will provide a guideline for clinicians managing ADRs associated with the COVID-19 vaccines and increase the confidence of the general public in the COVID-19 vaccines.
Efficacy of COVID-19 vaccines
To estimate the efficacy of the COVID-19 vaccines, we evaluated all the COVID-19 vaccine data that have been published from phase III clinical trials; a total of 194,015 cases were included. The overall efficacy was highly heterogeneous (>90%); therefore, we performed subgroup analyses with stratification by vaccine type, sex, and age, which effectively reduced the heterogeneity. The analysis of different types of vaccines showed that the efficacy of inactivated vaccines was 73.11% (95% confidence interval [CI], 34.23; 89.03), the efficacy of protein subunit vaccines was 89.33% (95% CI, 81.44; 93.10), and the efficacy of RNA-based vaccines was 94.29% (95% CI, 93.65; 95.40). The efficacy of the viral vector (non-replicating) vaccines was 79.56% (95% CI, 60.00; 89.92; Table1 ; Figure S2 ; Table S1 ).
The efficacy of COVID-19 vaccines
Since inactivated vaccines and protein subunit vaccines lacked subgroup data, including age and sex, only RNA-based vaccines and viral vector (non-replicating) vaccines were included in the subsequent subgroup analyses. Vaccine efficacy (VE) among male and female participants was 92.70% (95% CI, 81.00; 96.81) and 87.84% (95% CI, 75.78; 93.88), respectively. At the same time, the efficacy of vaccine among 16 to 55 years old recipients was 88.89% (95% CI, 75.45; 94.87) and that among those over 55 years old was 87.62% (95% CI, 76.83; 92.54). Only RNA-based vaccines and viral vector (non-replicating) vaccines provided the data of different races. In the subgroup analysis, VE among Black or African American and White participants was 95.37% (95% CI, 47.92; 100.00) and 89.81% (95% CI, 73.08; 96.15), respectively. We found that all vaccines achieved good efficacy, among which RNA-based vaccines had the highest, whereas inactivated vaccines had the lowest, although they were more than 70% effective. In addition, Black or African American people, males, and the 16- to-55-year-old subgroup experienced greater VE ( Table 1 ; Figure S2 ; Table S1 ).
Incidence of ADRs related to the COVID-19 vaccines
Safety is another important factor when considering vaccines. Therefore, we first performed a meta-analysis of the clinical trial data and then collected real-world data from the VAERS maintained by the CDC in the United States. In the clinical trials analysis, we evaluated a total of 6 phase III clinical trials and 6 phase I/II clinical trials and official reports of phase III results of COVID-19 vaccines, and 56,310 cases were included. Meanwhile, the data of 86,506,742 doses from 5 reports about the thromboembolic events were included, while 603,862,888 doses from 3 reports about the myocarditis/pericarditis events were included. In the real-world analysis, we included 11,936 participants. The results are as follows.
Incidence of ADRs in the meta-analysis of clinical trials
We observed 36 types of ADRs in the clinical trials, among which 8 were observed after vaccination with more than 50% of the vaccines ( Table S2 ), including pain, swelling, fever, fatigue, chills, muscle pain (myalgia), joint pain (arthralgia), and headache. We further conducted a meta-analysis of these 8 ADRs. Similarly, to minimize heterogeneity, we performed subgroup analyses stratified by dose, vaccine type, and age.
Since inactivated vaccines lacked ADRs data of dose 1, only RNA-based vaccines, viral vector (non-replicating) vaccines, and protein subunit vaccines were included in the analyses. The results showed that the most frequently reported ADR was pain (at the injection site) after dose 1 in protein subunit vaccines (38.46%) and RNA-based vaccines (80.97%). Pain was reported more frequently in younger vaccine recipients (16 to 55 years old) than in older vaccine recipients (over 55 years old; 80.00% versus 59.35%). Fatigue was the second most frequent ADR after dose 1 (30.77% of those receiving the protein subunit vaccines and 39.27% of those receiving the RNA-based vaccines). The incidence in the 16- to 55-year-old subgroup was significantly higher than that in the over 55-year-old subgroup (52.72% versus 33.73%). The incidences of other ADRs were below 50%. Headache ranked third, followed by muscle pain (myalgia), joint pain (arthralgia), chills, swelling, and fever. The incidence of ADRs after vaccination with RNA-based vaccines was high, and further analysis of age subgroups indicated that the results were generally consistent with those observed in the overall analysis. Meanwhile, unlike the two vaccines above, in viral vector (non-replicating) vaccines, the most frequently reported ADR was fatigue (56.25%). Headache ranked second, followed by pain, muscle pain (myalgia), joint pain (arthralgia), chills, fever, and swelling ( Table 2 ; Figures S3–S5 ; Table S2 ).
The incidence of ADRs associated with COVID-19 vaccines via meta-analysis
Among participants who received dose 2, the overall incidence of ADRs was higher than that after dose 1. As observed for dose 1, pain was the most frequent ADR. The incidences of pain in patients administered different types of vaccines were as follows: inactivated vaccines (31.75%), protein subunit vaccines (57.69%), RNA-based vaccines (81.76%), and viral vector (non-replicating) vaccines (44.75%). The incidences of pain in different age groups were as follows: 16 to 55 years old (72.40%) and over 55 years old (51.06%). The incidences of ADRs other than pain differed among the various types of vaccines. In particular, the incidence of ADRs was lowest for inactivated vaccines, with incidences of all ADRs less than 10%. In descending order of frequency, the ADRs were headache, swelling, fatigue, chills, joint pain (arthralgia), muscle pain (myalgia), and fever. The ADRs associated with the other three types of vaccines were similar to those after dose 1, with fatigue and headache ranking second and third, respectively. However, more than 50% of recipients experienced headache after dose 2, unlike after dose 1. Moreover, among the other ADRs with incidences less than 50%, chills ranked fifth after dose 2, while it had ranked sixth after dose 1, and the other ADRs in order were joint pain (arthralgia), swelling, and fever. For RNA-based vaccines and viral vector (non-replicating) vaccines, consistent results were obtained among subgroups stratified by age ( Table 2 ; Figures S7–S10 ; Table S2 ).
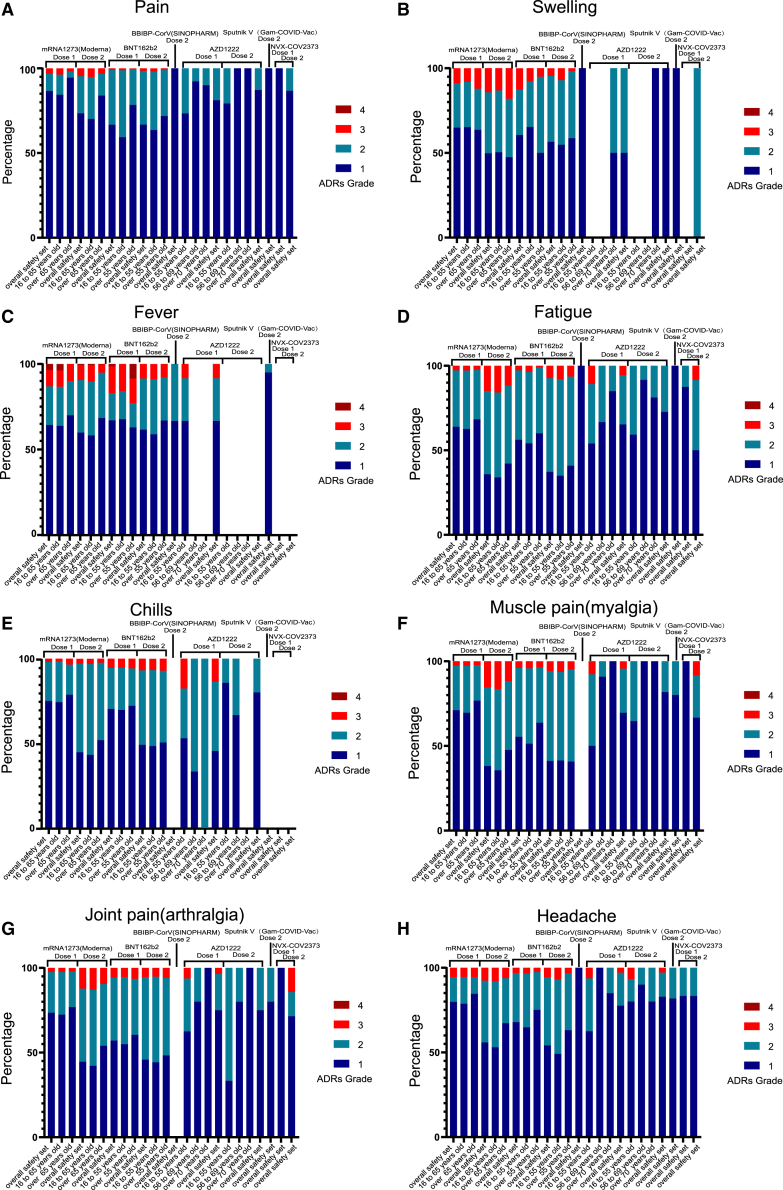
To assess the severity of vaccine-related ADRs, we calculated the proportions (the ADRs over grade 3/all ADRs) and conducted a meta-analysis according to severity grade. The results showed that the ADRs associated with the RNA-based vaccine (Moderna, BNT162b2) were the most severe, and instances of grade 3 reactions were reported for all 8 ADRs. Fortunately, the proportions were low, and even the largest was less than 20%. Grade 3 ADRs also occurred after vaccination with viral vector (non-replicating) vaccines (AZD1222, Sputnik V); however, the proportions were low (less than 10%). Most ADRs after vaccination with inactivated vaccines (BBIBP) and protein subunit vaccines (NVX-COV2373) were grades 1–2. Importantly, among the participants who received the first dose of an RNA-based vaccine, grade 4 fever was noted, but the proportion was less than 5%. Meanwhile, we also found that younger participants were more likely to report higher-grade ADRs than older participants. For the RNA-based vaccine (Moderna) and protein subunit vaccine (NVX-COV2373), the ADR grades were higher after the second dose than the first dose ( Figure 1 ; Table S2 ).
The severity of vaccine-related ADRs in clinical trials
Stacked bar chart showing the percentage of four ADRs grade after dose 1 or dose 2 of COVID-19 vaccines. (A) pain, (B) swelling, (C) fever, (D) fatigue, (E) chills, (F) muscle pain (myalgia), (G) joint pain (arthralgia), and (H) headache. Grade 1 (dark blue), grade 2 (light blue), grade 3 (red), and grade 4 (brown).
In the analysis of ADRs over grade 3, the incidences were all less than 10%, among which the most frequently reported ADR was fatigue (6.34%) in RNA-based vaccines after dose 2. The ADR grades were higher after the second dose than the first dose in RNA-based vaccines, contrary to the viral vector (non-replicating) vaccines (AZD1222, Sputnik V). What’s more, the incidences of the ADRs over grade 3 in viral vector (non-replicating) vaccines were higher than those in RNA-based vaccines after dose 1 ( Table 2 ; Figures S6 and S11 ; Table S2 ).
The severe and rare ADRs of COVID-19 vaccines
Besides the ones that have been reported in the clinical trials, there are some severe and rare ADRs, such as thromboembolic events and myocarditis/pericarditis events, which may result in death. Our results showed that thromboembolic events were only found in viral vector (non-replicating) vaccines (Ad26.COV2.S and AZD1222), while myocarditis/pericarditis events were reported in both viral vector (non-replicating) vaccines (Ad26.COV2.S and AZD1222) and RNA-based vaccines (BNT162b2 and Moderna). The incidence of thromboembolic events in Ad26.COV2.S (75 cases per million doses) was higher than that in AZD1222 (21 cases per million doses; Figure 2 A; Table S3 ). The incidence of myocarditis/pericarditis events was similar in viral vector (non-replicating) vaccines and RNA-based vaccines (2 versus 3 cases per million doses; Figure 2 B; Table S3 ).
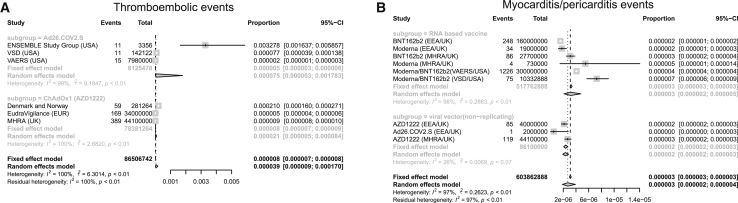
Forest plot of the incidence of thromboembolic events and myocarditis/pericarditis events
Meta-analysis was performed using R statistical software. Event rates and their corresponding 95% confidence intervals were estimated using both a fixed-effects model and a random-effects model. (A) Thromboembolic events and (B) myocarditis/pericarditis events.
Incidence of ADRs associated with RNA-based vaccines in the real world (VAERS)
To evaluate the safety of the COVID-19 vaccines more comprehensively, we retrospectively obtained real-world data pertaining to ADRs associated with RNA-based vaccines from VAERS. A total of 11,936 participants were included in the study, among whom 4,990 were vaccinated with the Moderna vaccine and 6,946 were vaccinated with the Pfizer-BioNTech vaccine ( Table S4 ).
Our research revealed an unexpected phenomenon. The incidence of ADRs in the real world was far lower than that in clinical trials. The ADR with the highest incidence is headache (16.53%), but the spectrum of ADRs is significantly wider than that in clinical trials. We identified more than 700 ADRs, but the incidence of most ADRs (more than 90%) was lower than 1% ( Figure 3 D). To evaluate the tolerance of the vaccine in different populations, we conducted subgroup analyses stratified by age, sex, and vaccine manufacturer. All ADRs with incidences higher than 5% were included. After stratification by the vaccine manufacturer (Moderna and Pfizer-BioNTech), the results showed that there were no significant differences in the incidences of headache, pain, myalgia, and nausea, but the incidences of chills, pyrexia, injection site pain, injection site erythema, pain in the extremities, and injection site swelling were higher among patients vaccinated with the Moderna vaccine than among those vaccinated with the Pfizer-BioNTech vaccine. In contrast, fatigue, dizziness, and dyspnea occurred more frequently in patients vaccinated with the Pfizer-BioNTech vaccine ( Figure 4 ; Table S6 ). The details of the incidences of all ADRs associated with the different vaccines are shown in Table S6 . Headache was still the most frequent ADR after the subgroup analysis was performed with stratification by age. Meanwhile, among those vaccinated with the Moderna vaccine, all ADRs were reported more often in older participants than young participants. The result was the opposite for the Pfizer-BioNTech vaccine ( Figures 3 D and 4 ; Tables S7 and S8 ). The details of the incidences of all ADRs in different age groups are shown in Tables S7 and S8 . In the analysis stratified by sex, we found that regardless of whether the Moderna or Pfizer-BioNTech vaccine was administered, pyrexia ranked first, which was different from the results of the other subgroup analyses. Other than pyrexia and chills, which were more common in males, the incidences of other ADRs were higher in females than in males ( Figures 3 D and 4 ; Tables S9 and S10 ). The details of the incidences of all ADRs stratified by sex are shown in Tables S9 and S10 .
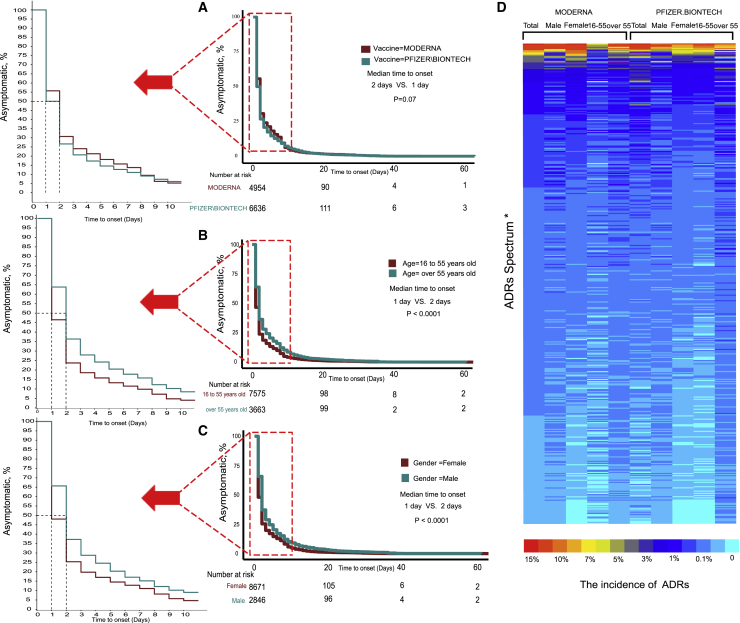
The incidence of ADRs of RNA-based vaccine from real-world data (VAERS)
Log-rank test of ADRs onset time stratified by (A) vaccine type, (B) age, and (C) gender. (D) Heatmap showing the incidence of ADRs. (∗ADRs Spectrum: due to the limitation of figure size, the details are shown in Table S5 .)
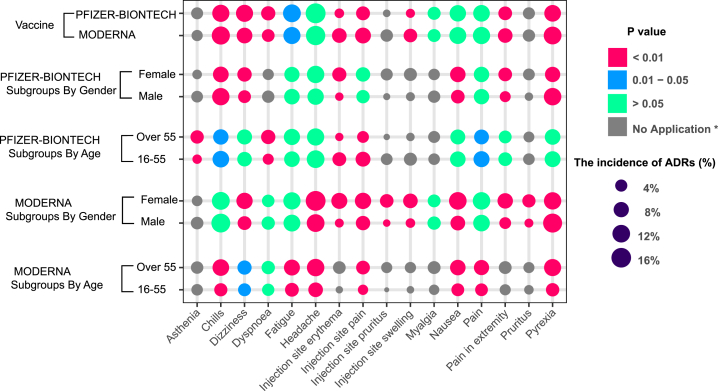
The subgroup analyses of ADRs in RNA-based vaccine from real-world data (VAERS)
To evaluate the tolerance of the vaccine in different populations, we conducted subgroup analyses stratified by age, sex, and vaccine manufacturer. All ADRs with incidences higher than 5% were included. (∗No application: the incidences of ADRs under 5% in the subgroups were defined as “no application,” which were not tested by χ 2 .)
We also further explored the timing of the onset of ADRs. Most participants developed symptoms within a week after vaccination, but the longest interval was 60 days. The median symptom onset time for the Moderna and Pfizer-BioNTech vaccines were 2 days and 1 day, respectively, but the difference was not statistically significant ( Figure 3 A, p = 0.07). Symptoms appeared earlier in young participants, and the median interval was 1 day, while in older people, it was 2 days ( Figure 3 B, p < 0.0001). Symptoms appeared earlier in females, with a median interval of 1 day, while in males it was 2 days ( Figure 3 C, p < 0.0001).
COVID-19 remains a global public health threat, although it has been more than a year since the first case was diagnosed. The number of cases and deaths from COVID-19 continues to increase. Undoubtedly, vaccines are the most promising means to control the COVID-19 pandemic. As of April 5, 2021, several vaccines had been approved for public use, including RNA-based vaccines (Moderna and Pfizer-BioNTech), inactivated vaccines (Sinopharm [BBIBP], CoronaVac, Covaxin, Sinopharm [WIBP], and CoviVac), viral vector vaccines (Oxford-AstraZeneca, Sputnik V, Johnson & Johnson, and Convidecia), and protein subunit vaccines (EpiVacCorona, RBD-Dimer). 3 Although vaccinations are continuing to be administered, the vaccinated population only accounts for a small proportion of the entire population, and safety and efficacy are the issues about which many people are concerned.
This is the first study on the efficacy and safety of COVID-19 vaccines using published clinical trial data and real-world data. We comprehensively analyzed the efficacy of the existing COVID-19 vaccines and their incidence, spectrum, timing, and clinical features of ADRs after vaccination. Our research indicated that the efficacy of all vaccines exceeded 70% and that RNA-based vaccines had the highest efficacy of 94.29%; moreover, young people, Black or African American people, and males may experience greater vaccine efficacy. The spectrum of vaccine-related ADRs is extremely broad, involving multiple systems. The most common ADRs are pain, fatigue, and headache. Most ADRs are tolerable and mainly in grade 1 or 2 in severity; only grade 4 fever has been observed. Some severe ADRs have been identified, though the incidences were low (thromboembolic events, 21–75 cases per million doses; myocarditis/pericarditis, 2–3 cases per million doses). Most symptoms appear soon after vaccination, and many people recover without any medication.
In terms of efficacy, RNA-based vaccines ranked first, reaching greater than 94%, due to their strong immunogenicity and effective presentation of SARS-CoV-2 antigens to the immune system. 7 Currently, mutant virus strains are also attracting attention. RNA-based vaccines may be more effective against these mutant strains owing to their use of the full immunogenicity of SARS-CoV-2. However, the incidence of ADRs is high after vaccination with RNA-based vaccines, reaching over 80% based on the clinical trial data, with the incidences of grade 3 or 4 ADRs accounting for a small proportion. Although the real-world incidence of ADRs was lower than that in the clinical trials, the spectrum was broader, and a large portion of types of ADRs were not observed in clinical trials, suggesting that attention should be given to the identification and treatment of rare ADRs. Meanwhile, myocarditis/pericarditis have been identified in RNA-based vaccines; fortunately, the incidence was low. Protein subunit vaccines had an efficacy of 89%, while the highest incidence of ADRs was only 57%, and highest incidence of the ADRs over grade 3 was 3.85%, significantly lower than that associated with RNA-based vaccines; therefore, it may be a promising candidate. However, because real-world data regarding protein subunit vaccines are lacking and the sample of published data is small, further analysis is needed. Moreover, viral vector (non-replicating) vaccines have an efficacy of 79%, while the highest incidence of ADRs is 40%. In addition, the incidence of ADRs above grade 3 is significantly lower than that associated with RNA-based vaccines. However, some thromboembolic events and myocarditis/pericarditis events have been reported after vaccination with viral vector (non-replicating) vaccines (Ad26.COV2.S and AZD1222), which are very severe. Fortunately, the incidences of thromboembolic events and myocarditis/pericarditis events were low. Inactivated vaccines, in particular, are very safe and easy to preserve and transport, although their efficacy is relatively lower.
In the subgroup analysis, the ADRs after dose 1 of viral vector (non-replicating) vaccine (AZD1222, Sputnik V) occurred more often than dose 2. In contrast, the incidence of ADRs was higher after dose 2 of the RNA-based vaccine produced by Moderna and the protein subunit vaccine called NVX-COV2373. The results suggest that there are differences among the vaccines, and the monitoring of ADRs cannot be taken lightly even if no adverse reaction occurs following dose 1, especially among those receiving RNA-based vaccines (e.g., Moderna) and protein subunit vaccines (e.g., NVX-COV2373). The second dose should not be avoided because of ADRs after dose 1. The process of building tolerance to viral vector (non-replicating) vaccines is gradual in vaccinated recipients. We also found that young people seem to be relatively more prone to higher grade ADRs. We speculate that the relatively stronger immune systems in young people lead to both a higher incidence of ADRs and greater vaccine efficacy. 8 This finding also reduces concerns about vaccinating elderly people. The higher incidence of ADRs among female participants than male participants is puzzling, because it suggests that a stronger immune response was elicited in females, but the efficacy is lower in females than in males. This is inconsistent with the results of previous studies on sex differences. 9 The specific reasons need to be explored further. Furthermore, in the analysis of the timing of the onset of ADRs, we found that young people and females developed symptoms earlier, which may be related to the higher incidence of ADRs and their stronger immune systems. 9 In addition, the interval between vaccination and the development of ADRs in some patients can be up to 60 days, suggesting that the vaccination history should be actively reported when symptoms develop after vaccination and clinicians should pay attention to the lag between vaccination and the development of ADRs.
In the ADR analysis, the real-world data from the VAERS and clinical trial data were compared. We found that there are differences in the spectrum of ADRs, with a wider spectrum of ADRs identified in the real-world data. One plausible explanation is that the data in VAERS are continuously and openly collected. However, only ADRs that occurred within 1 week were counted in most clinical trials, and those that appeared after 1 week were omitted. In addition, the VAERS system lacks a standardized description of symptoms, with multiple different descriptions referring to the same ADR, falsely increasing the spectrum of ADRs. Another surprising finding is that the incidence of ADRs in the real world is far lower than that in clinical trials. Real-world data are only available for RNA-based vaccines, and the sample size is not yet large enough. Additionally, the VAERS is a self-reporting system with reporting bias, 10 and a large number of participants who were vaccinated did not report their ADRs, resulting in a lower incidence rate than in clinical trials.
We also found that few cases of mortality were reported to VAERS, and there was not enough evidence to indicate that the death was related to vaccination after carefully assessing each case. Therefore, a large-scale real-world study is needed for further confirmation.
In addition to the possible bias in VAERS, our study also has other deficiencies. The heterogeneity of several subgroups was large in the meta-analysis. To minimize heterogeneity, we used a total of 5 transformation methods (PFT, PAS, PRAW, PLN, PLOGIT) and chose the method by which the lowest heterogeneity was achieved. 11
In addition, we also conducted sensitivity analyses and multiple subgroup analyses to minimize heterogeneity. Both fixed-effect model and random-effect model were performed. When I 2 was less than 50% and p > 0.1, the fixed-effect model was chosen; otherwise, the random-effect model was chosen. 12 , 13 , 14
The Begg’s and Egger’s tests were not used because there were not more than 10 subjects in each group. 15 Although some subgroups were heterogeneous, we determined that the heterogeneity was derived from the data itself after sufficient statistical correction and analysis, possibly due to factors such as the area in which the study was conducted, the risk of exposure to SARS-CoV-2, and other factors that were beyond our control. Therefore, our research comprehensively demonstrated the efficacy and safety of the COVID-19 vaccines to the greatest extent possible, providing a credible reference for clinical practice and the general public.
In summary, vaccines are a powerful tool against the COVID-19 pandemic, with high efficacy and tolerable adverse reactions. Each vaccine has its own advantages and shortcomings, and every citizen should choose to be vaccinated as soon as possible. In addition, the spectrum of ADRs associated with the vaccines is broad, and most of the reactions appear within a week, although a delay sometimes occurs. Some severe ADRs have been identified, though the incidences were low (thromboembolic events and myocarditis/pericarditis). Therefore, ADRs should be identified and addressed in a timely manner after vaccination. We hope that our research can eliminate fear of the vaccines among the general public and provide guidance regarding the management of vaccine-related side effects in a timely manner.
Materials and methods
Meta-analysis, part 1: the landscape of efficacy and safety of covid-19 vaccines, inclusion criteria.
The study was registered in PROSPERO (CRD42021234481). We identified records by searching PubMed, Medline, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) for “(COVID-19 OR 2019-nCoV OR SARS-CoV-2) AND vaccine” on March 7, 2021. English-language clinical trials were included.
Exclusion criteria
All 8,215 initially identified studies were screened; those that were clinical trials were included (n = 53), and those in which a vaccine against SARS-CoV-2 was not used were excluded (n = 29). Trials without adverse effect or efficacy data (n = 1) and those with only the clinical trial protocol (n = 1) were excluded.
The remaining trials (n = 22) included 17 phase I/phase II clinical trials of 12 vaccines, and 4 of these vaccines had published phase III clinical trial results (n = 5). We further searched for the remaining 8 vaccines on Google using the following keywords: “(candidate vaccine name or manufacturer) AND (COVID-19 OR 2019-nCoV OR SARS-CoV-2).” Phase I/phase II trials of vaccines that did not have official results from phase III clinical trials were excluded (n = 11).
The remaining trials (n = 11) included 8 different vaccines, phase III clinical trials were updated on June 17, 2021, and a new trial of Ad26.COV2.S vaccine was included (n = 1). Finally, 12 clinical trials were assessed individually, and a total of 194,015 cases were included. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 The total number of patients treated, the number and type of adverse effects, the VE were compared, and the PRISMA diagram of articles selected for meta-analysis was shown in Figure S1 ( Figure S1 A; Table 3 ).
Clinical trials and the characteristics of COVID-19 vaccines
Part 2: The severe and rare ADRs of COVID-19 vaccines
We identified records by searching PubMed, Medline, EMBASE, Google, and the CENTRAL for “(Thromboembolic OR Myocarditis OR Pericarditis) AND COVID-19 vaccine” on June 17, 2021. English-language clinical trials and official reports were included.
All 2,910,000 results initially identified studies were screened; those that were clinical trials (n = 1), cohort study (n = 3), case reports (n = 12), and official reports (n = 8) were included. Those studies without the data of the exact total number and exact number of patients with thromboembolic or myocarditis or pericarditis were excluded (n = 14). Those official reports that were outdated or without the data of the exact vaccine type (n = 3) were excluded.
The remaining trial (n = 1), 27 cohort study (n = 1), 28 and official reports (n = 5) 29 , 30 , 31 , 32 , 33 were assessed individually, and a total of 86,506,742 doses with thromboembolic events and 603,862,888 doses with myocarditis/pericarditis events were included. The total number of doses, the number and type of adverse effects, and the vaccine types were compared, and the PRISMA diagram of articles selected for meta-analysis is shown in Figure S2 ( Figure S1 B; Table S3 ).
The study is based on data downloaded from the VAERS ( https://vaers.hhs.gov/data.html ). The VAERS is comanaged by the CDC and the FDA and has been used to detect possible safety problems in U.S.-licensed vaccines since 1990. Healthcare providers, vaccine manufacturers, and the public can submit reports to the system. 6
We accessed the VAERS on March 5, 2021 and downloaded data from 2020 and 2021. We included all entries in which the patient had been injected with the Moderna or Pfizer COVID-19 vaccine. Patients injected with COVID-19 vaccines manufactured by unknown developers or vaccines against other pathogens were excluded.
VE was calculated as 1-relative risk (RR): 34 , 35
The incidence of ADRs was extracted by “Engauge Digitizer” from histograms if the raw data were not displayed. 36 The incidences of ADRs were compared with χ2 tests. Other clinical variables of interest were evaluated descriptively. Statistical analyses were performed in GraphPad Prism (version 7, GraphPad Software); the meta-analysis was performed using R statistical software (packages metafor and meta, R Foundation). Event rates and their corresponding 95% confidence intervals were estimated using both a fixed-effects model and a random-effects model. Forest plots were constructed to summarize the data for each analytical group according to the incidence rate and to provide a visual analysis of fatal drug-related events.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (number 2018YFC1313300), National Natural Science Foundation of China (numbers 81070362, 81172470, 81372629, 81772627, 81874073, and 81974384), key projects from the Nature Science Foundation of Hunan Province (numbers 2015JC3021 and 2016JC2037), the projects from Beijing CSCO Clinical Oncology Research Foundation (numbers Y-HR2019-0182 and Y-2019Genecast-043), and the Fundamental Research Funds for the Central Universities of Central South University University (2020zzts273 and 2019zzts797). We want to show our appreciates to Zirconicusso/Freepik for providing the materials for making the Graphical Abstract.
Author contributions
C.C., Y.P., H.S., S.Z., and Y.H. designed the study. C.C., Y.P., E.S., Q.H., Y.C., P.L., C.G., Z.F., L.G., Y.L., and X.Z. collected the data and performed the major analysis. S.Z. and H.S. supervised the study. C.C. and Y.P. analyzed and interpreted the data. E.S. and Z.F. did the statistical analysis. C.C., Y.P., C.G., and Y.L. drafted the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.08.001 .
Contributor Information
Ying Han, Email: [email protected].
Shan Zeng, Email: [email protected].
Hong Shen, Email: [email protected].
Supplemental information
- 1. Organization W.H. 2021. WHO Coronavirus (COVID-19) Dashboard. World Health Organization. https://covid19.who.int/ [ Google Scholar ]
- 2. Organization W.H. 2021. Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [ Google Scholar ]
- 3. Wikipedia . 2021. List of COVID-19 vaccine authorizations. https://en.wikipedia.org/w/index.php?title=List_of_COVID-19_vaccine_authorizations&oldid=1016045922 [ Google Scholar ]
- 4. Ritchie H., Ortiz-Ospina E., Beltekian D., Mathieu E., Hasell J., Macdonald B. 2021. Coronavirus (COVID-19) Vaccinations. https://ourworldindata.org/global-rise-of-education [ Google Scholar ]
- 5. Ontario P.H. 2021. COVID-19 – What we know so far about herd immunity. Toronto, ON: Queen’s Printer for Ontario. https://www.publichealthontario.ca/en/diseases-and-conditions/infectious-diseases/respiratory-diseases/novel-coronavirus/what-we-know [ Google Scholar ]
- 6. Chen R.T., Rastogi S.C., Mullen J.R., Hayes S.W., Cochi S.L., Donlon J.A., Wassilak S.G. The Vaccine Adverse Event Reporting System (VAERS) Vaccine. 1994;12:542–550. doi: 10.1016/0264-410x(94)90315-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Andrew M.K., McElhaney J.E. Age and frailty in COVID-19 vaccine development. Lancet. 2021;396:1942–1944. doi: 10.1016/S0140-6736(20)32481-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Goodman M.J., Nordin J. Vaccine adverse event reporting system reporting source: a possible source of bias in longitudinal studies. Pediatrics. 2006;117:387–390. doi: 10.1542/peds.2004-2687. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950;21:607–611. [ Google Scholar ]
- 12. Harris R.J., Deeks J.J., Altman D.G., Bradburn M.J., Harbord R.M., Sterne J.A. Metan: fixed-and random-effects meta-analysis. Stata J. 2008;8:3–28. [ Google Scholar ]
- 13. Gavaghan D.J., Moore A.R., McQuay H.J.J.P. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., Tonstad S., Vatten L.J., Riboli E., Norat T.J.b. 2016. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 353, i2716. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., Carpenter J., Rücker G., Harbord R.M., Schmid C.H.J.B. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., COVE Study Group Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Oxford COVID Vaccine Trial Group Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Gam-COVID-Vac Vaccine Trial Group Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Novavax I. 2021. Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. Accessed on March 21. https://ir.novavax.com/2021-01-28-Novavax-COVID-19-Vaccine-Demonstrates-89-3-Efficacy-in-UK-Phase-3-Trial [ Google Scholar ]
- 25. Bureau H.P.-F.a.H. 2021. Report on Evaluation of Safety, Efficacy and Quality of CoronaVac COVID-19 Vaccine (Vero Cell) Inactivated. Accessed on March 21. https://www.fhb.gov.hk/en/our_work/health/rr3.html [ Google Scholar ]
- 26. Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., ENSEMBLE Study Group Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., Lidegaard Ø., Tapia G., Gulseth H.L., Ruiz P.L.-D. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Tom Shimabukuro p. 2021. Thrombosis with thrombocytopenia syndrome(TTS) following Janssen COVID-19 vaccine. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-2004-2023/2003-COVID-Shimabukuro-2508.pdf [ Google Scholar ]
- 30. prevention C.f.d.c.a . 2021. COVID-19 Vaccine safety updates. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-2006/2003-COVID-Shimabukuro-2508.pdf [ Google Scholar ]
- 31. agency E.m . 2021. COVID-19 vaccines: update on ongoing evaluation of myocarditis and pericarditis. https://www.ema.europa.eu/en/news/covid-19-vaccines-update-ongoing-evaluation-myocarditis-pericarditis [ Google Scholar ]
- 32. Agency, M.H.p.R. 2021. Coronavirus vaccine - weekly summary of Yellow Card reporting. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting [ Google Scholar ]
- 33. agency E.m . 2021. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood [ Google Scholar ]
- 34. Nic Lochlainn L.M., de Gier B., van der Maas N., Strebel P.M., Goodman T., van Binnendijk R.S., de Melker H.E., Hahné S.J.M. Immunogenicity, effectiveness, and safety of measles vaccination in infants younger than 9 months: a systematic review and meta-analysis. Lancet Infect. Dis. 2019;19:1235–1245. doi: 10.1016/S1473-3099(19)30395-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Clark A., van Zandvoort K., Flasche S., Sanderson C., Bines J., Tate J., Parashar U., Jit M. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect. Dis. 2019;19:717–727. doi: 10.1016/S1473-3099(19)30126-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Shi X., Chen Q., Wang F. Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res. Ther. 2019;10:266. doi: 10.1186/s13287-019-1336-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (2.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The World of Immunization: Achievements, Challenges, and Strategic Vision for the Next Decade
Ann lindstrand, thomas cherian, diana chang-blanc, daniel feikin, katherine l o’brien.
- Author information
- Article notes
- Copyright and License information
Correspondence: Katherine L. O’Brien, MD MPH, World Health Organization, 20 Avenue Appia–CH-1211, Geneva 27, Switzerland ( [email protected] ).
Collection date 2021 Oct 1.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
Immunization is among the most cost-effective public health interventions available and is estimated to have averted at least 37 million deaths between 2000 and 2019. Since the establishment of the Expanded Programme on Immunization in 1974, global vaccination coverage increased and the coverage gap between rich and poor countries decreased. Creation of Gavi, the Vaccine Alliance, in 2000 allowed the poorest countries in the world to benefit from new, life-saving vaccines and expand the breadth of protection against an increasing number of vaccine-preventable diseases. Despite this progress, inequities in access to and uptake of vaccines persist. Opportunities to realize the full potential of vaccines are within reach but require focused, tailored and committed action by Governments and immunization stakeholders. The Immunization Agenda 2030 provides a framework for action during the next decade to attain a world where everyone, everywhere, at every age fully benefits from vaccines for good health and well-being.
Keywords: challenges, decade, EPI, history, IA2030, immunization, impact, overview, vaccines, vision
INTRODUCTION
Immunization is one of the most cost-effective public health preventive measures in countries of all income levels [ 1–4 ]. Six vaccine-preventable diseases constituted the initial focus of the Expanded Programme on Immunization (EPI) over 4 decades ago; at least 6 new vaccines have increased the breadth of protection provided by immunization, contributing to reductions in morbidity and mortality across the life course. In the midst of the challenges facing immunization programs during the COVID-19 pandemic, this article gives a historic overview of immunization, its achievements, current challenges, and introduces the new strategy and vision for the next decade, the Immunization Agenda 2030 (IA2030) [ 5 ].
Establishment of the Expanded Programme on Immunization
The global scaleup of immunization programs began with the establishment of EPI in 1974 by the World Health Assembly (WHA) [ 6 ]. The adoption of the resolution (WHA27.57) establishing EPI was made possible due to the successes achieved under the smallpox eradication program [ 6 ].
Initial progress was slow and by 1980, coverage with 3 doses of diphtheria-tetanus-pertussis (DTP)-containing vaccines (DTP3) globally was around 20% with a wide disparity in coverage between high- and low-income countries; DTP3 coverage in the poorest countries was 5% ( Figure 1 ). In response, in 1984, the Universal Childhood Immunization initiative led by the United Nations Children’s Fund (UNICEF) in collaboration with the World Health Organization (WHO) was established, with the ambitious aim of increasing DTP3 coverage to 80% globally by 1990. Not only did global DTP3 coverage nearly quadruple over the course of a decade, reaching 75% by 1990, but in the poorest countries rose from 5% in 1980 to 62% in 1990 ( Figure 1 ), substantially diminishing inequity with wealthy countries.
Three doses of diphtheria-tetanus-pertussis vaccine (DTP3) coverage by country income level, 1980 to 2019. World Health Organization United Nations Children’s Fund estimates of national immunization coverage.
The achievements of the EPI, along with the eradication of smallpox in 1980, laid the ground for launching aspirational vaccine-preventable disease goals over the next 20 years, including the eradication of poliomyelitis, elimination of maternal and neonatal tetanus, and elimination of measles and rubella in all WHO regions. Surveillance to document progress towards eradication and elimination goals led to the initiation and strengthening of communicable disease surveillance, especially in low- and middle-income countries (LMICs), which has served to detect and respond to epidemics of both vaccine-preventable and other communicable diseases for the past 40 years.
Immunization has contributed to Millennium Development Goal 4 to reduce childhood mortality. All-cause mortality in children younger than 5 years decreased by 47% between the year 2000 (9.7 million deaths) and 2019 (5.2 million deaths) [ 7 ]. Four of the top 10 causes contributing to this decrease are fully or partially vaccine-preventable (ie, pneumonia, diarrhea, measles, and meningitis; Figure 2 ) [ 8 ]. It is estimated that vaccination has averted at least 37 million deaths (95% credible interval 30–48) between 2000 and 2019, representing a 45% decline in deaths due to 10 vaccine-preventable diseases ( Haemophilus influenzae type b [Hib], Japanese encephalitis, Neisseria meningitidis serogroup A, measles, Streptococcus pneumoniae, rotavirus, rubella, and yellow fever) in 98 LMICs relative to no vaccination [ 9 ]. Additional deaths averted over a lifetime in the birth cohorts vaccinated from 2000 to 2030, including for hepatitis B and human papilloma virus (HPV), increase that to 120 million (93 to 150). The total annual global deaths averted by vaccination go far beyond these estimates as only 98 countries were modeled, and deaths averted from polio, diphtheria, tetanus, pertussis, and from adult vaccinations were not modelled [ 10 ]. In a related modeling study it was estimated that from 2011 to 2030, immunization would avert $1510.4 billion (2018 USD; 95% CL $674.3–$2643.2) in costs of illness in 94 LMIC compared with no vaccination, and generate $3436.7 billion (95% CL $1615.8–$5657.2) in benefits [ 11 ], representing a return of $26.1 for every dollar invested using the cost-of-illness approach [ 4 ].
Global numbers of child deaths preventable or partially preventable through vaccination, 1990–2017. Source: WHO, Global Health Observatory data, November 2018.
Gavi, the Vaccine Alliance
In the 1990s, immunization coverage stagnated and the slow introduction into lower-income countries of new life-saving vaccines, which were developed and implemented in many high-income countries (HICs), created serious inequities in protection from infectious disease threats to infant and child survival. In response, the Children’s Vaccine Initiative (CVI), an alliance of United Nations agencies, private foundations, and industry, was launched in 1990 with a vision that all children would be immunized and set 3 goals of vaccine supply, new vaccine development, and improved vaccine delivery [ 12 ]. However, the urgency to accelerate introduction of already available life-saving vaccines in low-income countries led to the dissolution of CVI in 1999 and the establishment of Gavi, the Vaccine Alliance (Gavi) in 2000. Founded by the Bill and Melinda Gates Foundation and key partners (WHO, UNICEF, and the World Bank), Gavi was established as a private-public partnership to catalyze the introduction of new and underused vaccines in the world’s poorest countries. Over the past 2 decades, financial and technical support from Gavi has led to the uptake of hepatitis B, Hib, yellow fever, pneumococcus, rotavirus, inactivated polio, and HPV vaccines in the world’s poorest countries, reducing the time lag between their introduction in high-income and in low-income settings.
Until 2020, Gavi approached country eligibility for support through the specification of economic status; those with per capita gross national income (GNI) below an established threshold over the preceding 3 years (according to the World Bank data published each July) can seek support for the introduction of new or underused vaccines, as well as for health systems strengthening funds for vaccine delivery infrastructure. Eligibility was set at an amount of US$1500 in 2011 and is updated annually to account for inflation [ 13 ]. Countries enter a transition process and start phasing out of Gavi support once their GNI exceeds the threshold. The policies for eligibility in the 2021–2025 period (Gavi 5.0) are being modified to account for the reality that many unimmunized children live in middle-income countries (MICs) and that an increasing number of children live in crisis-affected, fragile, and vulnerable settings.
The Global Vaccine Action Plan
The Global Vaccine Action Plan (GVAP) was a global strategy developed in response to a call in 2010 for a Decade of Vaccines [ 14 ]. GVAP was built upon the WHO and UNICEF Global Immunization and Vaccine Strategy (GIVS, 2006–2015), the first 10-year strategy for immunization. GVAP (2011–2020) expanded the partnership, provided a framework for achieving “a world in which all individuals and communities enjoy lives free from vaccine-preventable diseases,” and established measurable goals and targets for the decade [ 15 ]. The aspirational goals were to stop wild polio transmission; eliminate neonatal tetanus, measles, rubella, and congenital rubella syndrome; reach 90% national DTP3 coverage and 80% in every district; develop and introduce new vaccines and technologies; and reduce child mortality. One of the first global health strategies to be accompanied by a monitoring and accountability framework with an independent assessment of progress, GVAP achieved substantial progress.
PROGRESS WITH ACHIEVING GLOBAL GOALS
GVAP demonstrated that coordination and alignment of immunization partners are critical for attaining the aspirations of the decade. Even with these elements, attaining all goals remained elusive and only the target for introduction of new vaccines was met (goal 4) [ 16 ]. Nevertheless, enormous advances were achieved and form the basis for the next decade’s strategy.
Progress Towards Disease Eradication and Elimination
Through the global polio eradication efforts, cases of paralytic poliomyelitis decreased from 350 000 in 1988 to 140 in 2020, after touching a historic low of 22 cases in 2017 [ 17 ]. Of the 3 strains of wild poliovirus, type 2 was officially certified as globally eradicated in 2015 and type 3 in 2019 [ 17 ]. With the certification of the African continent as being free of all wild-type polio circulation in August 2020, only 2 countries, Pakistan and Afghanistan, continue to have endemic wild-type poliomyelitis transmission. Despite these impressive achievements, the final goal of eradication has remained stubbornly elusive. Recent hurdles have included an upswing in cases of wild-type 1 polio in Pakistan and Afghanistan (84 and 56 cases, respectively, in 2020) due to insecurity hampering routine and supplemental polio vaccination efforts.
Energized by the certification of the eradication of type 2 wild poliovirus in 2015, the Global Polio Eradication Initiative called for the withdrawal of oral polio vaccines starting with the type 2 component of oral polio vaccine (OPV2). This was achieved through a globally synchronized initiative referred to as the “Switch,” in which 156 countries and territories still using oral polio vaccine simultaneously switched from the use of trivalent OPV (tOPV; containing types 1, 2, and 3 poliovirus) to bivalent OPV (bOPV; containing types 1 and 3 poliovirus), in a 2-week time-period, thus removing OPV type 2 from routine use. The global Switch was an unprecedented endeavor and successful milestone for the program, and was accompanied by the introduction of at least 1 dose of inactivated polio vaccine (IPV) in the 126 countries that had an OPV-only schedule. However, since 2016, several challenges have confronted the polio program, including global IPV shortages that contributed to lower than desired coverage in countries where the vaccine was introduced, and months of delay in IPV introduction in many countries. Additionally, the emergence of circulating type 2 vaccine-derived poliovirus (cVDPV2) cases occurred quickly after the Switch, requiring expansive and repeated deployment of monovalent OPV2 from the global stockpile for outbreak response, which has reseeded type 2 poliovirus (vaccine derived) in communities with type 2 immunity gaps, creating a vicious cycle spreading and escalating cVDPVs over the past 2 years. In 2020, there were over 1087 AFP cases due to cVDPVs reported from 24 countries [ 18 ]. It is hoped that the implementation of the new cVDPV2 control strategy in early 2021—a key component of which is the roll-out of an improved version of monovalent OPV2 (novel OPV2) that is less likely to seed new outbreaks—will reverse this trend [ 19 ]. Despite its limitations, the lessons and experience from IPV introduction, which was the largest and fastest globally coordinated effort for vaccine introduction, has been leveraged for COVID-19 vaccine planning and introduction.
Prior to the introduction of measles vaccine in 1963, measles caused an estimated 2.6 million deaths annually [ 20 ]. This fell to 535 600 deaths by 2000; since then and through 2019, an estimated 25.5 million measles deaths have been averted by measles vaccination [ 21 ]. Estimated first-dose coverage of measles-containing vaccine increased globally from 72% to 84% over 2000 to 2010; however, between 2011 and 2019 it has plateaued at 84%–85%. Over 19 million children did not receive measles vaccination in 2019 [ 21 ]. The global number of reported measles cases (which represent a mere fraction of actual annual measles cases because of underreporting) more than quadrupled from 170 000 in 2017 to 863 000 in 2019, with several countries experiencing large outbreaks, most notably the Democratic Republic of Congo and Madagascar [ 22 ]. The Americas, the only WHO Region to verify the elimination of measles (in 2016), lost its elimination status in 2018 from reestablishment of endemic transmission that persisted for more than a 12-month period in some Latin American countries; the other 5 WHO regions have not achieved their time-bound elimination goals, which are now being reset.
The maternal and neonatal tetanus initiative, launched in 1999, targeted 59 endemic countries aiming to reduce neonatal tetanus incidence to < 1/1000 live births in every district of each country [ 23 ]. By 2019, 47 (80%) of the 59 target countries had achieved elimination and the estimated number of neonatal tetanus deaths decreased by 85%, from about 171 000 in 2000 to 25 000 in 2018 [ 23 ]. Neonatal tetanus afflicts the most marginalized populations, signaling that harsh societal and economic inequities still need to be overcome.
Immunization Coverage
DTP3 coverage is used as a marker of overall immunization program performance because DTP is universally recommended and coverage with 3 doses of DTP permits standardized monitoring over time and across countries. The massive equity gap between the rich and poor countries in 1980 has been markedly reduced; however, since 2000, DTP3 global coverage has remained stagnant between 84% and 86% ( Figure 1 ). In 2019, approximately 85% of infants worldwide (116 million infants) received 3 doses of DTP-containing vaccines [ 24 ]. However, the static global coverage masks a highly dynamic situation with impressive gains in some countries and troubling backsliding of coverage in others related to various issues including safety events and political, social, and economic turmoil [ 25 ]. There is no guarantee that immunization coverage gains of 1 year are sustained into the next. From a regional perspective, since 2015, DTP3 has steadily increased in the WHO African and South-East Asia regions while there has been a notable decline in coverage in the region of the Americas ( Figure 3 ). The increase in coverage in the African region is especially notable because of the growing birth cohort; to just maintain coverage has required substantial program expansion and increased performance ( Figure 4 ).
Three doses of diphtheria-tetanus-pertussis vaccine (DTP3) coverage by World Health Organization (WHO) region, 1980 to 2019. Abbreviations of WHO regions: AFR, Africa; AMR, the Americas; EMR, Eastern Mediterranean; EUR, Europe; SEAR, South-East Asia; WPR, Western Pacific.
Number of children (in millions) younger than 2 years with 3 doses of diphtheria-tetanus-pertussis vaccine (DTP3), dropout before DTP3, and unvaccinated with DTP1, by region 1980–2019. Abbreviations of World Health Organization regions: AFR, Africa; AMR, the Americas; EMR, Eastern Mediterranean; EUR, Europe; SEAR, South-East Asia; WPR, Western Pacific.
Introduction of New Vaccines
Since 2000, the number of vaccines included in childhood immunization schedules has increased dramatically. The historic time lag in the introduction of new vaccines between rich and poor countries has been shortened. Consequently, while DTP3 coverage has remained flat since 2000, the breadth of protection, reflecting the number of antigens and their coverage, has increased substantially ( Figure 5 ). Furthermore, immunization programs now include vaccinations beyond infancy to protect older age groups. These include provision of the second dose of measles-containing vaccines in the second year of life (MCV2) or later, booster doses of DTP vaccine in preschool and school-age children, the introduction of HPV vaccines in the preadolescent and adolescent age group, and seasonal influenza, pneumococcal, and herpes zoster vaccines in older adults, and now COVID-19 vaccines. As of 2019, 178 countries were providing MCV2 in the second to fifth year of life, 106 were providing HPV to preadolescent and adolescent girls, and 112, 25, and 10 were providing seasonal influenza, pneumococcal, and herpes zoster vaccination, respectively, to older adults [ 26 ]. Pregnant women are an increasing focus for vaccination, as a means to protect their vulnerabilities and to transplacentally transfer immunity to protect their newborn infants. This advances not only vaccination programs for pregnant women against tetanus, influenza, and pertussis for which recommendations exist, but also advances the interests of pregnant women in vaccine research, especially for emerging diseases [ 27–28 ]. Nevertheless, the roll-out of new vaccines has been uneven. Introduction of rubella vaccines was delayed due to concerns about a paradoxical increase in congenital rubella in countries with suboptimal coverage, provision of a timely birth dose of hepatitis B vaccine has been impeded in communities where a sizeable proportion of births occur outside the health facility, and the introduction of HPV vaccines is likely to be affected by vaccine supply shortfalls.
Changes in the breadth of vaccination coverage, 2000 to 2019. The global average coverage for 13 antigens provided through national immunization programs (World Health Organization United Nations Children’s Fund Estimates of National Immunization Coverage). Abbreviations: Hep B, hepatitis B; Hib, Haemophilus influenzae type b; HPV, human papilloma virus; IPV, inactivated polio vaccine; Pneumo, pneumococcus; Rota, rotavirus.
FACTORS CONTRIBUTING TO SUCCESS
Many factors contributed to the achievements in immunization over the past decades. Improvements in delivery infrastructure; vaccine-preventable disease surveillance and the requisite regional and global lab networks; ever growing community engagement and advocacy on the value of vaccines; more sophisticated information and social media platforms; electronic data collection, management, and use; vaccine research and development capacity; expansion and coordination of vaccine regulatory and safety monitoring systems; increasingly data-driven policy decision making at the country level; and global partnership all underpin this progress. Here we highlight a few factors that are less scientifically driven, yet essential to fully realize the promise of vaccines.
Commitments by National Governments
National government commitment to prioritizing immunization programs is widely recognized as one of the most impactful success factors without which other efforts falter. Many LMICs have substantially enhanced their commitments to immunization. In 2011, national health ministers in the WHO South-East Asia region expressed their commitment through the Delhi Call for Action to intensify the drive toward achieving high routine immunization coverage throughout the region [ 29 ]. Similarly, in 2017, the heads of states of countries in the African continent endorsed the Addis Declaration at the 28th African Union Summit, pledging to ensure that everyone in Africa—regardless of who they are or where they live—receives the full benefits of immunization [ 30 ]. The commitments of national governments are reflected by increases in national expenditures on immunization. These increased by 35% in 2016–2017 compared to 2010–2011; they were greatest in the African (74%), South-East Asian (62%), and Western Pacific regions (75%) [ 31 ]. Countries also demonstrated their commitment by establishing and/or substantially strengthening national immunization technical advisory groups (NITAGs) to ensure that policymaking on the use of vaccines was driven by evidence. NITAGs meeting all the WHO functionality criteria nearly tripled, from 41 in 2010 to 114 in 2018 [ 16 ]. Furthermore, all regions now have Regional Immunization Technical Advisory Groups to adapt global policies to regional contexts, and support NITAGs in adapting and applying these policies at the national level.

Affordable Pricing and Sustained Supply of Vaccines
Ensuring an adequate supply of safe and effective vaccines remains a global priority in a world with unprecedented changes in the vaccine industry and market [ 32–34 ]. LMIC manufacturers have an increasing role in vaccine supply, and their entrance into the market is stimulating price declines of key vaccines [ 35 ]. More than 60% of the original 6 EPI vaccines are supplied by LMIC manufacturers [ 36 ], who are increasingly entering into development or technology transfer agreements with multinational manufacturers [ 37 ].
Predictable financing and innovative procurement mechanisms managed through UNICEF, Gavi, and the Pan American Health Organization Revolving Fund have further contributed to successful tiered pricing mechanisms and substantially reduced pricing for newer vaccines. The weighted average price of the pentavalent vaccine (containing diphtheria, tetanus, pertussis, hepatitis B, and Hib vaccines) for Gavi countries declined from US$2.98 in 2010 to US$0.79 in 2019 [ 16 ]. The Vaccine Product, Price and Procurement (V3P) project, and a web-based platform on Market Information for Access (MI4A) have improved price transparency and sustainable access to vaccines at affordable prices [ 38 ].
Improvements in Supply Chains and Logistics
Supply chain and logistics systems that strive to ensure the uninterrupted availability of high-quality and potent vaccines up to the point of administration are the delivery backbone to a well-functioning immunization system, as referred to in WHO’s Immunization Practice Advisory Committee 2014 call to action [ 39 ]. The regular monitoring of vaccine stock levels to mitigate stock-outs and address root causes has reduced the risk of interruptions in vaccination services and consequent decline in vaccination coverage in many countries [ 40 ]. While few LMICs currently meet the minimum performance standard for every dimension at all levels of the immunization supply chain, as measured through the WHO and UNICEF Effective Vaccine Management assessment process [ 41 ], investments by national governments for strengthening their supply chain and establishing robust logistic management information systems are gradually growing.
Strategies to Reach the Unimmunized
While strong service delivery systems are essential for achieving high and equitable vaccination coverage, these systems need to be accompanied by appropriate policies and strategies that promote the equitable and timely delivery of vaccination. The WHO Global Routine Immunization Strategies and Practices (GRISP) lays out in a comprehensive manner 9 transformative investments that enable resilient programs [ 42 ]. GRISP promotes approaches that go beyond conventional service delivery through health facilities and outreach services for detecting and reaching marginalized and partially served communities. These include the Periodic Intensification of Routine Immunization, a mechanism to catch-up individuals who may have missed their routine doses [ 43 ]. The WHO Reaching Every District strategy aims to support countries in achieving at least 80% immunization coverage in every district and at least 90% nationally through improved microplanning at the district level [ 44 ]. Reducing missed opportunities for vaccination by using all contacts between an individual and the health system is another approach that is being adopted by countries to improve vaccination coverage across the life course [ 45 ].
ADDRESSING THE CHALLENGES FOR GLOBAL IMMUNIZATION
While there has been measurable progress in the current decade, several challenges have impeded progress. Additional efforts, leveraging available opportunities, are needed to realize the new global vision and impact goals of the IA2030 and achieve the 2030 Sustainable Development Goals [ 46 ].
Stagnant and Inequitable Coverage
Achieving and sustaining high and equitable vaccination coverage is fundamental to securing the greatest impact possible with existing and new vaccines. Despite progress in several countries that have had longstanding low vaccination coverage, many have difficulties in equitably reaching and sustaining the 90% target. Several issues merit consideration. In 2019, 20 million children were un- or underimmunized with DTP3, and 14 million of these did not even receive the first dose of DTP, referred to as “zero dose” children [ 25 ]. These children are not randomly distributed around the world; over 60% of them are in just 10 countries, some of which have low vaccination coverage (eg, Nigeria and Angola) while others have high coverage but large birth cohorts contributing a large number of undervaccinated children (eg, India and Indonesia). Within countries, these children are from families and communities most likely also to be left out from other essential health services. They are disproportionately those who are impoverished, in rural areas and urban slums, or live in settings of conflict, fragility, or vulnerability [ 47 ].
Policies Lacking an Equity Focus
Existing legislation, policies, and guidelines may inadvertently create or exacerbate inequities. Policies that limit services to individuals registered with civil authorities in a country or region can create barriers for refugee, mobile, or migrant populations, excluding them from access to public health services, including immunization. Gender strongly influences achieving immunization program goals in many settings. A myriad of social and policy structures, such as the ability of women to seek immunization services without having to be accompanied, the gender of immunization workers going door to door, or services that also meet the health needs of women coming for their child’s immunization services are all examples. Examining existing laws and policies through an equity lens will reveal opportunities to address inequities through enactment of new legislation, revision of legal frameworks, and establishment of new policies [ 48 ].
Human Resource Gaps
The positive correlation between health care worker density and immunization coverage is well documented [ 49–51 ]. Nevertheless, public health stakeholders and many governments have been unsuccessful in resolving their health worker shortages. Solutions are not straight forward; considerable time, effort, and money are required to train and retain health workers. As immunization programs increase in complexity, the skills and competencies of health workers must also develop. WHO’s Standard Competencies Framework for the Immunization Workforce provides guidance for countries to strengthen human resource capacity to manage and implement their programs [ 52 ].
Inadequate and Unpredictable Financing
The addition of new, more expensive vaccines to EPI has led to increases in the cost of a full vaccination course for a child since 1974; in the last decade alone, the average global immunization expenditures per live birth has increased by 50% [ 31 ]. Access to sufficient and predictable financing is essential for immunization programs to sustain vaccination coverage, service quality, and access to newer vaccines [ 15 ]. Although national governments in LMICs are increasing the allocations of domestic resources for their immunization programs, dependency on external support is projected to continue in the coming years [ 53 ]. In countries benefiting from Gavi support, absolute government investments have increased, but government financing accounted for only 37% of total immunization expenditures in 2017, which represents a 46% decline compared to 2010 because expenditures have outpaced absolute increases in domestic financing [ 40 ].
While government health budgets will remain the primary sources of immunization financing, new financing mechanisms need to be explored if LMICs are to sustain their immunization programs. As part of their efforts towards achieving universal health coverage, several new mechanisms are being used or considered for the costs of delivering an essential package of health services. An immunization financing resource guide provides options for raising additional resources to support immunization programs and the use of social insurance to partly finance immunization programs [ 54 ].
Uneven Access to Vaccines at Affordable Prices
The uptake of new vaccines in MICs that are not eligible for Gavi support has been slower than in Gavi-eligible countries [ 55 ]. In fact, coverage of pneumococcal and rotavirus vaccines is now higher in Gavi countries than the global average because many MICs have yet to introduce these vaccines ( Figure 6 ). Available information suggests that there is a wide range of prices paid by MICs for the same vaccine products. Previous studies have identified inefficient procurement as a barrier to competitive prices for vaccines [ 56 ]. While the market intelligence and price transparency provided by the WHO’s V3P and MI4A platforms have made an important contribution, improvements to country regulatory procedures and procurement practices, which contribute to higher pricing, has been patchy and more is required to secure affordable price access.
Trends in coverage with pneumococcal conjugate and rotavirus vaccines by country income levels and eligibility for Gavi support. Coverage in each country group is the product of the number of countries in the group that have introduced the vaccine and the coverage achieved nationally.
Limitations in the Quality and Use of Immunization and Surveillance Data
A major obstacle to closing current immunization gaps has been the lack of timely and high-quality data to inform decision making, operational planning, performance management of immunization staff, and service delivery. This information is essential at all levels of the health system for the effective delivery of immunization services. The absence of such data has consequences including missed opportunities to identify under- or unimmunized persons, inadequate defaulter tracking, and burdensome data collection processes that divert health provider time and attention away from the provision of high-quality service delivery. WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) highlighted these issues and recommended specific actions to improve the quality and use of data [ 57 ].
Coverage Monitoring
Inaccuracies in vaccination coverage estimates result from imprecise estimates of the target population size (the denominator) and errors in the recording of immunization doses administered (the numerator). In many LMICs, estimates of the target population size are from national census data, which are often very outdated. Despite the well-documented benefits of civil registration and vital statistic systems (CRVS), countries have been slow to adopt these systems. WHO guidelines and tools are available to conduct comprehensive assessment of CRVS and to strengthen them [ 58 ]. Several LMICs have established national plans based on these comprehensive assessments.
An increasing number of LMICs are leveraging the availability of new information and communication technologies to update their immunization data management systems that would improve the recording, timely transmission, and use of data to monitor their programs. Electronic immunization registries (EIRs) are computerized, confidential, population-based systems that contain individual-level information on vaccine doses administered [ 59 , 60 ]. An increasing number of high and middle-income countries have, or are establishing, EIRs [ 59–61 ], while a few LMICs have received support to establish or pilot EIRs [ 62 ].
Many other LMICs are using the District Health Information Software 2 (DHIS2), an open source, web-based health management information system platform, to collect and manage health and immunization data [ 63 ]. DHIS2 has developed an e-tracker that enables collection, management, and analysis of transactional, case-based data records, supports storage of information about individuals, and tracks these persons over time using a flexible set of identifiers. This system was rolled out in Rwanda in 2019 as an EIR [ 62 ]. It has recently been adapted to support the COVID-19 vaccine introduction monitoring, including a safety reporting module.
Disease and safety surveillance
Surveillance is required to measure disease burden, quantify the impact of immunization programs in reducing vaccine-preventable diseases, and identify changes in disease epidemiology that may require modifications of vaccination schedules and/or adaptation of program strategies. Disease surveillance data are also key for informing policy decisions and supporting sustained financing for the immunization program. The surveillance systems in many LMICs remain fragmented into disease-specific surveillance initiatives largely dependent on external resources. A shift to country-owned, comprehensive disease surveillance is needed to address the fragmentation, improve efficiencies, and ultimately lead to sustainable surveillance as is laid out in WHO’s new global strategy for comprehensive vaccine-preventable disease surveillance [ 64 ].
Funding for disease surveillance activities has leaned heavily on the Global Polio Eradication Initiative for many years and is expected to wind down over this decade. Alternate funding, including more country-level commitment to surveillance, will be needed. An investment case for surveillance in the African region indicates that if current vaccine-preventable disease surveillance and laboratory networks are enhanced, there would be an estimated 45-fold return on investment by 2030 [ 65 ].
Safety monitoring and surveillance for adverse events following immunization are key components of a strong immunization program. The WHO Global Vaccine Safety Blueprint aims to optimize the safety of vaccines through effective use of pharmacovigilance principles and methods and assist low and LMICs to have at least minimal capacity for vaccine safety activities [ 66 ].
Wavering Community Demand for Vaccination
It is naive to assume that if health services are made accessible to communities, that health-seeking behavior will follow [ 67 ]. Analysis of data reported to WHO and UNICEF for 2015 to 2017 showed that vaccine hesitancy, defined as “delay in acceptance or refusal of vaccination despite availability of vaccination services,” was common; over 90% of countries reported encountering hesitancy in accepting vaccination [ 68 ]. This led WHO to include vaccine hesitancy among 10 threats to global health in 2019 [ 69 ]. There were varied reasons cited for hesitancy with heterogeneity across countries [ 68 ]. A recent review found that while confidence in vaccines fell in some countries, it improved in others, highlighting the need for continuous monitoring and corrective actions [ 70 ]. However, demand for immunization encompasses more than hesitancy alone. It is also misleading to draw a clear distinction between service- or system-side factors and demand-related issues because an individual’s prior experience with the health system may influence their future demand or uptake of services [ 67 ]. Given the highly contextual nature of vaccine demand, community engagement and formative research is required to identify the determinants of demand and tailor services to improve the uptake of vaccination at local levels. The Tailoring Immunization Programs [ 71 ] and Human Centered Design for Health [ 67 ] are tools to enable structured, adaptable and participatory processes to target undervaccinated or hesitant populations using behavioral insights to understand the barriers and enablers of vaccination and to design, implement, and evaluate tailored, gender-responsive interventions to address them [ 72 ]. The Vaccination Demand Hub is a network of partner organizations collaborating to understand why people miss out and to improve acceptance and uptake of vaccines through actions to counter the priority barriers [ 73 ].
Emerging Inequities in Middle-Income Countries
MICs account for 60% of global deaths in children younger than 5 years, and a similar or greater share of vaccine-preventable deaths and unvaccinated children [ 74 ]. The initial impetus for the focus on MICs in the immunization field was a concern that these countries, excluded from or facing the loss of donor support and insufficient domestic investments in health, may be missing out on opportunities to introduce important new vaccines. However, the problems facing MICs extend far beyond the introduction of newer vaccines. Globally, vaccination coverage in the fully self-financing MICs has declined in recent years while coverage in HICs remained stable and increased in Gavi-supported LMICs ( Figure 1 ). In the European region, the MICs not eligible for Gavi support are lagging behind the HICs and the Gavi-supported MICs in progress with other regional goals [ 75 ]. A strategy to support fully self-financing MICs exists but needs the support and resources for its implementation [ 74 ].
Outbreaks, Conflicts, and Humanitarian Emergencies
The COVID-19 pandemic has demonstrated that immunization systems need to have the resilience to rapidly recover from acute shocks, such as during prolonged disease outbreaks and other causes of disruption. Lessons need to be documented from the Global Polio Eradication Initiative and case studies developed from exemplar countries who have overcome the challenges posed by conflict, civil unrest, and other humanitarian emergencies to cultivate best practices on sustaining immunization services during periods of severe disruption. In addition, countries need to improve their preparedness for and capacity to rapidly detect and respond to outbreaks of vaccine-preventable diseases to mitigate the risks of a prolonged epidemic. Conversely, data from outbreaks should be used to identify root causes behind immunity gaps, to address both vaccine supply- and demand-side shortcomings.
Impact of the COVID-19 Pandemic
The COVID-19 pandemic has revealed the vulnerability of the immunization program, an essential health service. In the first quarter of 2020, sharp declines in immunization coverage, ranging from 10% to 50% relative to 2019, occurred as severe social and physical distancing policies were implemented in many countries around the world ( Figure 7 ). During this same period, preventive polio, measles, and other vaccine campaigns were temporarily suspended due to the concern for COVID-19 transmission in campaign settings. Cumulatively as of 15 October 2020, 91 vaccination campaigns in 53 countries had been postponed including for polio, measles, rubella, tetanus, diphtheria, cholera, typhoid, meningitis, and yellow fever. Within 3 months of the onset of vaccine program disruptions, countries sought and implemented adaptations and innovations with the aim to restore and recover essential immunization services, including for outbreak response and preventive campaigns. New ways of working, such as offering immunization services in novel locations, drive-through vaccination, expanding service days and hours, providing appointment times rather than open clinic hours, and leveraging social media have all mitigated the impact of the pandemic. As a result, several regions showed a V-shaped recovery in vaccination service delivery ( Figure 7 ). By December 2020, 57 vaccination campaigns that had been postponed were implemented. Nevertheless, as countries phase out of lock-down measures, implementation of aggressive catch-up strategies will be required to immunize those who have missed their scheduled immunizations, to prevent risks of vaccine-preventable disease outbreaks such as measles [ 76 ].
Impact of the COVID-19 pandemic on routine childhood immunization. Relative difference between DTP3 doses administered in 2020–2021 and the similar time period in 2019 in 5 of the 6 WHO regions. Abbreviations: AFR, Africa; AMR, Americas; COVID-19, coronavirus disease 2019; DTP3, 3 doses of diphtheria-tetanus-pertussis vaccine; EMR, Eastern Mediterranean; SEAR, South-East Asia; WHO, World Health Organization; WPR, Western Pacific. Source: monthly administrative data on third dose of DTP administered reported to WHO as of 4 March 2021.
LOOKING TO THE FUTURE
A covid-19 vaccines roll out.
An unprecedented worldwide effort began in January 2020 to develop safe and effective COVID-19 vaccines to help end the COVID-19 pandemic that has impacted the lives and livelihoods of people worldwide. Following interim phase 3 clinical trial results and authorization for emergency use, COVID-19 vaccines began rollout in December 2020. This is an unprecedented landmark in immunization and shows how this pandemic has created a paradigm shift in the process, speed and scale vaccines are being developed, deployed, and financed. The goal of facilitating fair and equitable access to COVID-19 vaccines has motivated the establishment of the COVAX Global Vaccine Facility as the vaccine pillar of the Access to COVID Tools Accelerator. The Facility, co-led by WHO, Gavi, and the Coalition for Epidemic Preparedness Innovations in partnership with UNICEF and others, aims to assure speed, scale, and equitable access to COVID-19 vaccines with demonstrated efficacy and safety [ 77 ].
The WHO target product profile for COVID-19 vaccines provides a benchmark of the minimum characteristics needed for the assessment of candidates [ 78 ]. There are around 264 vaccine candidates in development as of 21 March 2021 of which 20 are in or entering phase 3 clinical trials [ 79 ]. An allocation framework for equitable distribution of vaccines across countries has been developed by WHO in consultation with its member states [ 80 ]. A values framework that articulates the ethical principles through which vaccine allocation and prioritization should be made, and a roadmap for prioritization of limited vaccine supply within countries to target populations, has been issued by SAGE and endorsed by WHO [ 81 , 82 ]. SAGE is providing product specific recommendations for any COVID-19 vaccine with emergency use listing by WHO or authorization by a WHO-recognized stringent regulatory authority.
Leveraging the Power of Research and Innovation
New technologies and innovative strategies have the potential to increase the reach and impact of immunization programs. While research has led to the development of new life-saving vaccines, research to develop new or improved products better suited to local needs, implementation research on how to optimize immunization among underserved populations, and to develop, evaluate, and scale up innovations for immunization service delivery could amplify the impact of available products as well as those in the pipeline. One example of targeting innovations to local needs is The Vaccine Innovation Prioritization Strategy, which undertook a formal process, including engaging in-country stakeholders, to prioritize 3 vaccine product innovations (among 24) with the greatest potential to achieve equity, improve immunization systems, and focus investment; these 3 innovations are microarray patches, heat-stable controlled temperature chain liquid formulations, and barcoding on primary containers [ 83 ]. However, research needs to extend beyond the development and launch of innovative products to address managerial, systems, sociobehavioral, financial, and communications bottlenecks. Implementation and operational research is one of the key focus areas in the IA2030 [ 5 ].
Catalyzing the Move Towards Life-Course Vaccination
Immunization beyond the infant schedule has not yet reached the scale of implementation it merits. The reasons to build strong immunization services throughout the life course are many [ 84 ]. First, to deliver booster and missed doses to achieve durable individual protection and herd immunity, and thereby achieve better disease control. Second, for protection against diseases with higher risk of transmission or more severe morbidity during pregnancy, in health care workers, travelers, in older ages, or for medical risk groups. To establish and maintain life-course immunization, policy and legal frameworks need to be adapted, systems to monitor immunization in older age groups expanded, and collaborations to integrate age-appropriate and catch-up vaccination into public and private health services strengthened. COVID-19 vaccine delivery will be an opportunity to strengthen adult vaccination approaches and integration with other health care programs, because the target groups include health care workers, adults of older age, and medical risk groups.
The Immunization Agenda 2030
In August 2020, all WHO member states endorsed the Immunization Agenda 2030: A Global Strategy to Leave No One Behind (IA2030) [ 5 ]. IA2030 envisions “a world where everyone, everywhere, at every age, fully benefits from vaccines to improve health and well-being.” It was developed in a co-creative process with close engagement of countries to ensure that the vision, strategic priorities, and goals are aligned with country needs. During its development, collective input came from over 60 organizations and in-person global and regional workshops of more than 750 individuals. The agenda is organized into 7 strategic priorities for immunization in the next decade: immunization programs for primary health care and universal health coverage; demand and commitment; coverage and equity; life course and integration; outbreaks and emergencies; supply and financing; and research and innovation. Four core principles—people-centered, country-owned, partnership, and data-guided—underpin all strategic priorities and are essential to its success.
IA2030 focuses on tailored implementation and adaptive approaches to country contexts and is built to be flexible to new challenges throughout the decade. The main shift from GVAP is a move away from disease-specific ways of operating and place immunization as an integral part of strong people-centered primary health care service. The agenda places countries at the center, supported by accountable partners including those outside the health sector. Other shifts include the importance of data-driven program and policy improvements, and the importance placed on targeted ways of addressing inequities and gender-related barriers. Measles being the most contagious vaccine-preventable disease, is recognized as a pathfinder for IA2030, signaling when and where immunization services need to be improved. To operationalize IA2030, regional IA2030 plans are being developed and countries are integrating the strategies into national immunization strategies aligned with the public health plans in each country. The monitoring and accountability framework is being developed through a wide country consultation process and will be presented to the World Health Assembly in May 2021. The IA2030 offers a valuable platform for sustaining global commitment and promoting greater accountability for improved health and well-being.
Acknowledgments. The authors gratefully acknowledge the contributions of the following staff from the Department of Immunization, Vaccines, and Biologicals and Global Polio Eradication Initiative, WHO, Geneva, and from the Immunization Departments at the WHO Regional Offices in providing information and data for preparing the manuscript: Sébastian Antoni, Andre Arsene Bita Fouda, Sunil Bahl, Nyambat Batmunkh, Kelly Carr, Natasha Crowcroft, Carolina Danovaro, Siddhartha Datta, Laure Dumolard, Kamal Fahmy, Marta Gacic-Dobo, Randie Gibson, Jan Grevendonk, Santosh Gurung, Quamrul Hasan, Lee Lee Ho, Katrina Kretsinger, Ondrej Mach, Balcha Girma Masresha, Richard Mihigo, Yohann Nedelec, Roberta Pastore, Alain Nyembo Poy, Alejandro Ramirez Gonzalez, Cuauhtemoc Ruiz, Mohammed Sharifuzzaman, Anita Shet, Claudia Steulet, Yoshihiro Takashima, Martha Velandia, and Simona Zipursky.
Disclaimer. The named authors alone are responsible for the views expressed in this publication.
Supplement sponsorship. This supplement is sponsored by the Bill and Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
- 1. Miller MA, Hinman AR. Economic analysis of vaccine policies. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. Philadelphia, PA: Elsevier Saunders, 2013:1413–26. [ Google Scholar ]
- 2. Bloom DE. The value of vaccination. Adv Exp Med Biol 2011; 697:1–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff (Millwood) 2016; 35:199–207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Sim SY, Watts E, Constenla D, Brenzel L, Patenaude BN. Return on investment from immunization against 10 pathogens in 94 low- and middle-income countries, 2011–30. Health Aff (Millwood) 2020; 39:1343–53. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. World Health Organization. Immunization agenda 2030: a global strategy to leave no one behind. https://www.who.int/immunization/immunization_agenda_2030/en/ . Accessed 15 September 2020.
- 6. Okwo-Bele JM, Cherian T. The expanded programme on immunization: A lasting legacy of smallpox eradication. Vaccine 2011; 29(Suppl 4):D74–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. UN Interagency Group for Child Mortality Estimation (IGME). Under-five mortality rate-total. https://childmortality.org/data/World . Accessed 12 October 2020.
- 8. Liu L, Hill K, Oza S, et al. . Levels and causes of mortality under age five years. In: Black RE, Laxminarayan R, Temmerman M, Walker N, eds. Reproductive, maternal, newborn, and child health: disease control priorities. 3rd ed. Vol 2. Washington, DC: World Bank, 2016. [ Google Scholar ]
- 9. Li X, Mukandavire C, Cucunuba ZM, et al. . Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet 2021; 397:398–408. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Cohen AL, Patel MK, Cherian T. Vaccines work: a reason for celebration and renewed commitment. Lancet 2021; 397:351–3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Watts E, Sim SY, Constenla D, Sriudomporn S, Brenzel L, Patenaude B. Economic benefits of immunization for 10 pathogens in 94 low- and middle-income countries from 2011 to 2030 using cost-of-illness and value-of-statistical-life approaches. Value Health 2021; 24:78–85. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Caddell A. The children’s vaccine initiative. Afr Health 1997; 20:15. [ PubMed ] [ Google Scholar ]
- 13. Gavi, the Vaccine Alliance. Gavi Alliance eligibility and transition policy, version 3. https://www.gavi.org/sites/default/files/document/gavi-eligibility-and-transition-policypdf.pdf . Accessed 26 February 2021.
- 14. Moxon ER, Das P, Greenwood B, et al. . A call to action for the new decade of vaccines. Lancet 2011; 378:298–302. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Global vaccine action plan 2011–2020. Geneva: World Health Organization, 2013. [ Google Scholar ]
- 16. Strategic Advisory Group of Experts on Immunization. The global vaccine action plan 2011–2020. Review and lessons learned. Geneva: World Health Organization, 2019. https://apps.who.int/iris/bitstream/handle/10665/329097/WHO-IVB-19.07-eng.pdf?ua=1 . Accessed 1 July 2021. [ Google Scholar ]
- 17. World Health Organization. Poliomyelitis (polio). https://www.who.int/health-topics/poliomyelitis#tab=tab_1 . Accessed 2 September 2020. [ Google Scholar ]
- 18. Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus. https://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/ . Accessed 21 March 2021.
- 19. Polio Global Eradication Initiative. GPEI strategy for the response to cVDPV2 2020–2021. http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf . Accessed 12 October 2020.
- 20. World Health Organization. Measles. https://www.who.int/news-room/fact-sheets/detail/measles . Accessed 02 September 2020.
- 21. Patel MK, Goodson JL, Alexander JP, et al. . Progress toward regional measles elimination—worldwide, 2000–2019. MMWR Morb Mortal Wkly Rep 2020; 69:1700–5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. World Health Organization. Provisional monthly measles and rubella data. https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/surveillance/monitoring/provisional-monthly-measles-and-rubella-data . Accessed 2 July 2021.
- 23. Njuguna HN, Yusuf N, Raza AA, Ahmed B, Tohme RA. Progress toward maternal and neonatal tetanus elimination—worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep 2020; 69:515–20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. World Health Organization. Immunization coverage: key facts. https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage . Accessed 27 August 2020.
- 25. World Health Organization. Progress and challenges with achieving universal immunization coverage: 2019 WHO/UNICEF estimates of national immunization coverage. https://www.who.int/immunization/monitoring_surveillance/who-immuniz.pdf?ua=1 . Accessed 27 August 2020.
- 26. World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2020 global summary immunization schedule selection centre. https://apps.who.int/immunization_monitoring/globalsummary/schedules . Accessed 27 August 2020.
- 27. Krubiner CB, Faden RR, Karron RA, et al. . Pregnant women and vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine 2021; 39:85–120. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Krubiner CB, Faden RR. Pregnant women should not be categorised as a ‘vulnerable population’ in biomedical research studies: ending a vicious cycle of ‘vulnerability’. J Med Ethics 2017; 43:664–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. World Health Organization. Intensification of routine immunization. https://www.who.int/southeastasia/activities/intensification-of-routine-immunization . Accessed 27 August 2020.
- 30. WHO Regional Office for Africa. Addis declaration on immunization. https://www.afro.who.int/health-topics/immunization/the-addis-declaration-immunization . Accessed 27 August 2020.
- 31. World Health Organization. Situational analysis of immunization expenditure: key facts. https://www.who.int/immunization/programmes_systems/financing/data_indicators/situation_Analysis_key-facts.pdf?ua=1 . Accessed 12 October 2020.
- 32. Greco M. Key drivers behind the development of global vaccine market. Vaccine 2001; 19:1606–10. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Maggon K. Industrial R&D paradigm shift to vaccines. Biotechnol J 2009; 4:458–61. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Vandersmissen W. WHO expectation and industry goals. Vaccine 2001; 19:1611–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Gupta SS, Nair GB, Arora NK, Ganguly NK. Vaccine development and deployment: opportunities and challenges in India. Vaccine 2013; 31(Suppl 2):B43–B53. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Milstien JB, Kaddar M. The role of emerging manufacturers in access to innovative vaccines of public health importance. Vaccine 2010; 28:2115–21. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Pagliusi S, Makhoana M, Datla M, et al. . Developing countries vaccine manufacturers network (DCVMN): engaging to step up for vaccine discovery and access. Meeting report 2012. Vaccine 2013; 31:3111–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. World Health Organization. Vaccine markets. https://www.who.int/teams/immunization-vaccines-and-biologicals/vaccine-access/mi4a . Accessed 2 July 2021.
- 39. World Health Organization. Immunization supply chain and logistics: a call to action. https://www.who.int/immunization/call-to-action_ipac-iscl.pdf . Accessed 12 October 2020.
- 40. World Health Organization. Global vaccine action plan monitoring, evaluation and accountability. Secretariat annual report 2018. https://www.who.int/immunization/global_vaccine_action_plan/web_gvap_secretariat_report_2018.pdf?ua=1 . Accessed 3 July 2021.
- 41. World Health Organization. Effective Vaccine Management (EVM). https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/supply-chain/effective-vaccine-management-(evm) . Accessed 2 July 2021.
- 42. World Health Organization. Global Routine Immunization Strategies and Practices (GRISP). https://www.who.int/publications/i/item/global-routine-immunization-strategies-and-practices-(grisp) . Accessed 2 July 2021. [ Google Scholar ]
- 43. World Health Organization. Periodic intensification of routine immunization. http://www.who.int/immunization/programmes_systems/policies_strategies/piri_020909.pdf?ua=1 . Accessed 28 May 2015.
- 44. World Health Organization. Microplanning for immunization service delivery using the reaching every district (RED) strategy. https://apps.who.int/iris/bitstream/handle/10665/70450/WHO_IVB_09.11_eng.pdf;jsessionid=858434B0071FE23C09A8267B7B9E486D?sequence=1 . Accessed 3 September 2020.
- 45. World Health Organization. Methodology for the assessment of missed opportunities for vaccination. https://www.who.int/publications/i/item/9789241512954 . Accessed 2 July 2021.
- 46. Gavi, the Vaccine Alliance. Immunisation and the sustainable development goals. https://www.gavi.org/sites/default/files/document/2019/Immunisation%20and%20the%20SDGs%20%281%29.pdf . Accessed 21 March 2021.
- 47. Chopra M, Bhutta Z, Chang Blanc D, et al. . Addressing the persistent inequities in immunization coverage. Bull World Health Organ 2020; 98:146–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. WHO Regional Office for Europe. Policy coherence as a driver of health equity. https://apps.who.int/iris/bitstream/handle/10665/324736/9789289054119-eng.pdf?sequence=1&isAllowed=y . Accessed 27 August 2020.
- 49. Hu Y, Shen L, Guo J, Xie S. Public health workers and vaccination coverage in eastern China: a health economic analysis. Int J Environ Res Public Health 2014; 11:5555–66. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Kruk ME, Prescott MR, de PH, Galea S. Are doctors and nurses associated with coverage of essential health services in developing countries? A cross-sectional study. Hum Resour Health 2009; 7:27. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Mitchell AD, Bossert TJ, Yip W, Mollahaliloglu S. Health worker densities and immunization coverage in Turkey: a panel data analysis. Hum Resour Health 2008; 6:29. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. World Health Organization. Workforce competencies. https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/training/workforce-competencies . Accessed 2 July 2021.
- 53. Gandhi G, Lydon P, Cornejo S, Brenzel L, Wrobel S, Chang H. Projections of costs, financing, and additional resource requirements for low- and lower middle-income country immunization programs over the decade, 2011–2020. Vaccine 2013; 31(Suppl 2):B137–48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Results for Development. Immunization financing: a resource guide for advocates, policymakers, and program managers. https://wayback.archive-it.org/13606/ 20200313235937/https://immunizationfinancing.org/home/Immunization_Financing_Resource_Guide_2017_FULL.pdf . Accessed 20 June 2020.
- 55. World Health Organization. Global Vaccine Action Plan: monitoring, evaluation and accountability secretariat annual report 2018. https://www.who.int/immunization/global_vaccine_action_plan/web_gvap_secretariat_report_2018.pdf?ua=1 . Accessed 15 October 2019. [ Google Scholar ]
- 56. Makinen M, Kaddar M, Molldrem V, Wilson S. New vaccine adoption in lower-middle-income countries. Health Policy Plan 2012; 27:ii39–ii49. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Meeting of the strategic advisory group of experts on immunization, October 2019: conclusions and recommendations. Wkly Epidemiol Rec 2019; 94:541–60. [ PubMed ] [ Google Scholar ]
- 58. World Health Organization. Strengthening civil registration and vital statistics for births, deaths and causes of death. https://www.who.int/publications/i/item/strengthening-civil-registration-and-vital-statistics-for-births-deaths-and-causes-of-death Accessed 2 July 2021.
- 59. Danovaro-Holliday MC, Contreras MP, Pinto D, et al. . Assessing electronic immunization registries: the Pan American Health Organization experience. Rev Panam Salud Publica 2019; 43:e28. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Derrough T, Olsson K, Gianfredi V, et al. . Immunisation information systems - useful tools for monitoring vaccination programmes in EU/EEA countries, 2016. Euro Surveill 2017; 22:30519. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Wu W, Cao L, Zheng J, Cao L, Cui J, Xiao Q. Immunization information system status in China, 2017. Vaccine 2019; 37:6268–70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Pancholi J, Birdie R, Guerette J, Chritz S, Sampath V, Crawford J. Landscape analysis of electronic immunization registries; lessons learned from a landscape analysis of EIR implementations in low and middle income countries. https://www.villagereach.org/wp-content/uploads/ 2020/06/2020-EIR-Landscape-Analysis.pdf . Accessed 31 August 2020.
- 63. World Health Organization. Report of the SAGE working group on quality and use of immunization and surveillance data. Revision September 2019. https://www.who.int/immunization/sage/meetings/2019/october/5_SAGE_report-revSept2019.pdf?ua=1 . Accessed 01 June 2020.
- 64. World Health Organization. Global strategy on comprehensive vaccine-preventable disease surveillance. IA2030. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/BLS20116_IA_Global_strategy.pdf?ua=1 . Accessed 03 September 2020.
- 65. WHO Regional Office for Africa. Investment case for vaccine-preventable diseases surveillance in the African Region 2020–2030. https://www.afro.who.int/sites/default/files/2019-11/VPD_Surv_Brochure_Final_20190918_WEB.pdf . Accessed 02 September 2020.
- 66. World Health Organization. Global vaccine safety blueprint. https://apps.who.int/iris/bitstream/handle/10665/70919/WHO_IVB_12.07_eng.pdf;jsessionid=D59F5DC3F9FDD553E6D62CAB4B849A24?sequence=1 . Accessed 15 October 2019.
- 67. Hickler B, MacDonald NE, Senouci K, Schuh HB, Informal Working Group on Vaccine D , Strategic Advisory Group of Experts on Immunization Working Group on Decade of Vaccines. Efforts to monitor global progress on individual and community demand for immunization: development of definitions and indicators for the Global Vaccine Action Plan Strategic Objective 2. Vaccine 2017; 35:3515–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF joint reporting form data-2015–2017. Vaccine 2018; 36:3861–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. World Health Organization. Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 . Accessed 27 February 2021.
- 70. de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet 2020; 396:898–908. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. World Health Organization. Tailoring immunization programmes (TIP). https://www.euro.who.int/en/health-topics/ disease-prevention/vaccines-and-immunization/activities/tailoring-immunization-programmes-tip . Accessed 10 August 2020.
- 72. Habersaat KB, Jackson C. Understanding vaccine acceptance and demand-and ways to increase them. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020; 63:32–9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. The Vaccination Demand Hub. https://www.demandhub.org/ . Accessed 27 February 2021.
- 74. World Health Organization. Sustainable access to vaccines in middle-income countries (MICs): a shared partner strategy. Report of the WHO-convened MIC task force. https://www.who.int/immunization/sage/meetings/2015/april/Cernuschi_MIC_Strategy_SAGE_Apr2015.pdf?ua=1 . Accessed 14 May 2019.
- 75. WHO Regional Office for Europe. European vaccine action plan 2015–2020. Midterm report. https://www.euro.who.int/__data/assets/pdf_file/0007/381184/evap-midterm-report-eng.pdf . Accessed 30 August 2020.
- 76. World Health Organization. Immunization as an essential health service: guiding principles for immunization activities during times of severe disruption, including during the COVID-19 pandemic. https://www.who.int/immunization/sage/meetings/2020/october/Session02A_GuidingPrinciplesImmunizationServices.pdf?ua=1 . Accessed 25 October 2020.
- 77. World Health Organization. ACT-A prioritized strategy and budget for 2021. who.int/publications/m/item/act-a-prioritized-strategy-and-budget-for-2021 . Accessed 21 March 2021.
- 78. World Health Organization. WHO target product profiles for COVID-19 vaccines. https://www.who.int/blueprint/priority-diseases/key-action/WHO_Target_Product_Profiles_for_COVID-19_web.pdf . Accessed 12 October 2020.
- 79. World Health Organization. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines . Accessed 12 October 2020.
- 80. World Health Organization. Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility. https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility . Accessed 12 October 2020.
- 81. World Health Organization. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. https://apps.who.int/iris/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf?ua=1 . Accessed 12 October 2020.
- 82. World Health Organization. Roadmap for prioritizing population groups for vaccines against COVID-19. https://www.who.int/immunization/sage/meetings/2020/october/Session03_Roadmap_Prioritization_Covid-19_vaccine.pdf?ua=1 . Accessed 12 October 2020.
- 83. Gavi, the Vaccine Alliance. The vaccine innovation prioritisation strategy (VIPS). https://www.gavi.org/our-alliance/market-shaping/vaccine-innovation-prioritisation-strategy . Accessed 12 October 2020.
- 84. Teresa Aguado M, Barratt J, Beard JR, et al. . Report on WHO meeting on immunization in older adults: Geneva, Switzerland, 22–23 March 2017. Vaccine 2018; 36:921–31. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2021
Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation
- Sarah Kreps 1 ,
- Nabarun Dasgupta 2 ,
- John S. Brownstein 3 , 4 ,
- Yulin Hswen 5 &
- Douglas L. Kriner ORCID: orcid.org/0000-0002-9353-2334 1
npj Vaccines volume 6 , Article number: 73 ( 2021 ) Cite this article
20k Accesses
83 Citations
43 Altmetric
Metrics details
- Health care
- Translational research
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still undermine efforts to combat the pandemic. Employing a survey of 1096 adult Americans recruited via the Lucid platform, we examined the relationships between vaccine attributes, proposed policy interventions such as financial incentives, and misinformation on public vaccination preferences. Higher degrees of vaccine efficacy significantly increased individuals’ willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use Authorization to fast-track the vaccine decreased willingness. The vaccine manufacturer had no influence on public willingness to vaccinate. We also found no evidence that belief in misinformation about COVID-19 treatments was positively associated with vaccine hesitancy. The findings have implications for public health strategies intending to increase levels of community vaccination.
Similar content being viewed by others

Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA
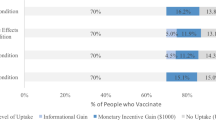
Vaccine hesitancy and monetary incentives
Providing normative information increases intentions to accept a COVID-19 vaccine
Introduction.
In less than a year, an array of vaccines was developed to bring an end to the SARS-CoV-2 pandemic. As impressive as the speed of development was the efficacy of vaccines such as Moderna and Pfizer, which are over 90%. Despite the growing availability and efficacy, however, vaccine hesitancy remains a potential impediment to widespread community uptake. While previous surveys indicate that overall levels of vaccine acceptance may be around 70% in the United States 1 , the case of Israel may offer a cautionary tale about self-reported preferences and vaccination in practice. Prospective studies 2 of vaccine acceptance in Israel showed that about 75% of the Israeli population would vaccinate, but Israel’s initial vaccination surge stalled around 42%. The government, which then augmented its vaccination efforts with incentive programs, attributed unexpected resistance to online misinformation 3 .
Research on vaccine hesitancy in the context of viruses such as influenza and measles, mumps, and rubella, suggests that misinformation surrounding vaccines is prevalent 4 , 5 . Emerging research on COVID-19 vaccine preferences, however, points to vaccine attributes as dominant determinants of attitudes toward vaccination. Higher efficacy is associated with greater likelihood of vaccinating 6 , 7 , whereas an FDA Emergency Use Authorization 6 or politicized approval timing 8 is associated with more hesitancy. Whether COVID-19 misinformation contributes to vaccine preferences or whether these attributes or policy interventions such as incentives play a larger role has not been studied. Further, while previous research has focused on a set of attributes that was relevant at one particular point in time, the evidence and context about the available vaccines has continued to shift in ways that could shape public willingness to accept the vaccine. For example, governments, employers, and economists have begun to think about or even devise ways to incentivize monetarily COVID-19 vaccine uptake, but researchers have not yet studied whether paying people to receive the COVID-19 vaccine would actually affect likely behavior. As supply problems wane and hesitancy becomes a limiting factor, understanding whether financial incentives can overcome hesitancy becomes a crucial question for public health. Further, as new vaccines such as Johnson and Johnson are authorized, knowing whether the vaccine manufacturer name elicits or deters interest in individuals is also important, as are the corresponding efficacy rates of different vaccines and the extent to which those affect vaccine preferences. The purpose of this study is to examine how information about vaccine attributes such as efficacy rates, the incidence of side effects, the nature of the governmental approval process, identity of the manufacturers, and policy interventions, including economic incentives, affect intention to vaccinate, and to examine the association between belief in an important category of misinformation—false claims concerning COVID-19 treatments—and willingness to vaccinate.
General characteristics of study population
Table 1 presents sample demographics, which largely reflect those of the US population as a whole. Of the 1335 US adults recruited for the study, a convenience sample of 1100 participants consented to begin the survey, and 1096 completed the full questionnaire. The sample was 51% female; 75% white; and had a median age of 43 with an interquartile range of 31–58. Comparisons of the sample demographics to those of other prominent social science surveys and U.S. Census figures are shown in Supplementary Table 1 .
Vaccination preferences
Each subject was asked to evaluate a series of seven hypothetical vaccines. For each hypothetical vaccine, our conjoint experiment randomly assigned values of five different vaccine attributes—efficacy, the incidence of minor side effects, government approval process, manufacturer, and cost/financial inducement. Descriptions of each attribute and the specific levels used in the experiment are summarized in Table 2 . After seeing the profile of each vaccine, the subject was asked whether she would choose to receive the vaccine described, or whether she would choose not to be vaccinated. Finally, subjects were asked to indicate how likely they would be to take the vaccine on a seven-point likert scale.
Across all choice sets, in 4419 cases (58%) subjects said they would choose the vaccine described in the profile rather than not being vaccinated. As shown in Fig. 1 , several characteristics of the vaccine significantly influenced willingness to vaccinate.
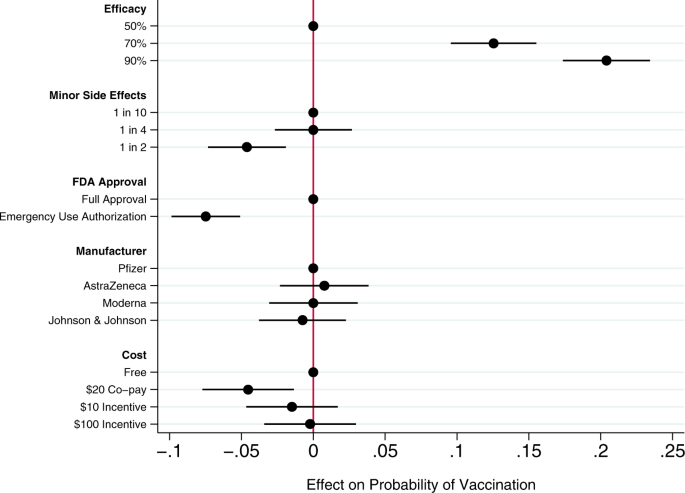
Circles present the estimated effect of each attribute level on the probability of a subject accepting vaccination from the attribute’s baseline level. Horizontal lines through points indicate 95% confidence intervals. Points without error bars denote the baseline value for each attribute. The average marginal component effects (AMCEs) are the regression coefficients reported in model 1 of Table 3 .
Efficacy had the largest effect on individual vaccine preferences. An efficacy rate of 90% increased uptake by about 20% relative to the baseline at 50% efficacy. Even a high incidence of minor side effects (1 in 2) had only a modest negative effect (about 5%) on willingness to vaccinate. Whether the vaccine went through full FDA approval or received an Emergency Use Authorization (EUA), an authority that allows the Food and Drug Administration mechanisms to accelerate the availability and use of treatments or medicines during medical emergencies 9 , significantly influenced willingness to vaccinate. An EUA decreased the likelihood of vaccination by 7% compared to a full FDA authorization; such a decline would translate into about 23 million Americans. While a $20 co-pay reduced the likelihood of vaccination relative to a no-cost baseline, financial incentives did not increase willingness to vaccinate. Lastly, the manufacturer had no effect on vaccination attitudes, despite the public pause of the AstraZeneca trial and prominence of Johnson & Johnson as a household name (our experiment was fielded before the pause in the administration of the Johnson & Johnson shot in the United States).
Model 2 of Table 3 presents an expanded model specification to investigate the association between misinformation and willingness to vaccinate. The primary additional independent variable of interest is a misinformation index that captures the extent to which each subject believes or rejects eight claims (five false; three true) about COVID-19 treatments. Additional analyses using alternate operationalizations of the misinformation index yield substantively similar results (Supplementary Table 4 ). This model also includes a number of demographic control variables, including indicators for political partisanship, gender, educational attainment, age, and race/ethnicity, all of which are also associated with belief in misinformation about the vaccine (Supplementary Table 2 ). Finally, the model also controls for subjects’ health insurance status, past experience vaccinating against seasonal influenza, attitudes toward the pharmaceutical industry, and beliefs about vaccine safety generally.
Greater levels of belief in misinformation about COVID-19 treatments were not associated with greater vaccine hesitancy. Instead, the relevant coefficient is positive and statistically significant, indicating that, all else being equal, individuals who scored higher on our index of misinformation about COVID-19 treatments were more willing to vaccinate than those who were less susceptible to believing false claims.
Strong beliefs that vaccines are safe generally was positively associated with willingness to accept a COVID-19 vaccine, as were past histories of frequent influenza vaccination and favorable attitudes toward the pharmaceutical industry. Women and older subjects were significantly less likely to report willingness to vaccinate than men and younger subjects, all else equal. Education was positively associated with willingness to vaccinate.
This research offers a comprehensive examination of attitudes toward COVID-19 vaccination, particularly the role of vaccine attributes, potential policy interventions, and misinformation. Several previous studies have analyzed the effects of vaccine characteristics on willingness to vaccinate, but the modal approach is to gauge willingness to accept a generic COVID-19 vaccine 10 , 11 . Large volumes of research show, however, that vaccine preferences hinge on specific vaccine attributes. Recent research considering the influence of attributes such as efficacy, side effects, and country of origin take a step toward understanding how properties affect individuals’ intentions to vaccinate 6 , 7 , 8 , 12 , 13 , but evidence about the attributes of actual vaccines, debates about how to promote vaccination within the population, and questions about the influence of misinformation have moved quickly 14 .
Our conjoint experiment therefore examined the influence of five vaccine attributes on vaccination willingness. The first category of attributes involved aspects of the vaccine itself. Since efficacy is one of the most common determinants of vaccine acceptance, we considered different levels of efficacy, 50%, 70%, and 90%, levels that are common in the literature 7 , 15 . Evidence from Phase III trials suggests that even the 90% efficacy level in our design, which is well above the 50% threshold from the FDA Guidance for minimal effectiveness for Emergency Use Authorization 16 , has been exceeded by both Pfizer’s and Moderna’s vaccines 17 , 18 . The 70% efficacy threshold is closer to the initial reports of the efficacy of the Johnson & Johnson vaccine, whose efficacy varied across regions 19 . Our analysis suggests that efficacy levels associated with recent mRNA vaccine trials increases public vaccine uptake by 20% over a baseline of a vaccine with 50% efficacy. A 70% efficacy rate increases public willingness to vaccinate by 13% over a baseline vaccine with 50% efficacy.
An additional set of epidemiological attributes consisted of the frequency of minor side effects. While severe side effects were plausible going into early clinical trials, evidence clearly suggests that minor side effects are more common, ranging from 10% to 100% of people vaccinated depending on the number of doses and the dose group (25–250 mcg) 20 . Since the 100 mcg dose was supported in Phase III trials 21 , we include the highest adverse event probability—approximating 60% as 1 in 2—and 1 in 10 as the lowest likelihood, approximating the number of people who experienced mild arthralgia 20 . Our findings suggest that a the prevalence of minor side effects associated with recent trials (i.e. a 1 in 2 chance), intention to vaccinate decreased by about 5% versus a 1 in 10 chance of minor side effects baseline. However, at a 25% rate of minor side effects, respondents did not indicate any lower likelihood of vaccination compared to the 10% baseline. Public communications about how to reduce well-known side effects, such as pain at the injection site, could contribute to improved acceptance of the vaccine, as it is unlikely that development of vaccine-related minor side effects will change.
We then considered the effect of EUA versus full FDA approval. The influenza H1N1 virus brought the process of EUA into public discourse 22 , and the COVID-19 virus has again raised the debate about whether and how to use EUA. Compared to recent studies also employing conjoint experimental designs that showed just a 3% decline in support conditional on EUA 6 , we found decreases in support of more than twice that with an EUA compared to full FDA approval. Statements made by the Trump administration promising an intensely rapid roll-out or isolated adverse events from vaccination in the UK may have exacerbated concerns about EUA versus full approval 8 , 23 , 24 , 25 . This negative effect is even greater among some subsets of the population. As shown in additional analyses reported in the Supplementary Information (Supplementary Fig. 5 ), the negative effects are greatest among those who believe vaccines are generally safe. Among those who believe vaccines generally are extremely safe, the EUA decreased willingness to vaccinate by 11%, all else equal. This suggests that outreach campaigns seeking to assure those troubled by the authorization process used for currently available vaccines should target their efforts on those who are generally predisposed to believe vaccines are safe.
Next, we compared receptiveness as a function of the manufacturer: Moderna, Pfizer, Johnson and Johnson, and AstraZeneca, all firms at advanced stages of vaccine development. Vaccine manufacturers in the US have not yet attempted to use trade names to differentiate their vaccines, instead relying on the association with manufacturer reputation. In other countries, vaccine brand names have been more intentionally publicized, such as Bharat Biotech’s Covaxin in India and Gamaleya Research Institute of Epidemiology and Microbiology Sputnik V in Russia. We found that manufacturer names had no impact on willingness to vaccinate. As with hepatitis and H. influenzae vaccines 26 , 27 , interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research suggests that at least as far as public receptiveness goes, interchangeability would not introduce concerns. We found no significant differences in vaccination uptake across any of the manufacturer treatments. Future research should investigate if a manufacturer preference develops as new evidence about efficacy and side effects becomes available, particularly depending on whether future booster shots, if needed, are deemed interchangeable with the initial vaccination.
Taking up the question of how cost and financial incentives shape behavior, we looked at paying and being paid to vaccinate. While existing research suggests that individuals are often willing to pay for vaccines 28 , 29 , some economists have proposed that the government pay individuals up to $1,000 to take the COVID-19 vaccine 30 . However, because a cost of $300 billion to vaccinate the population may be prohibitive, we posed a more modest $100 incentive. We also compared this with a $10 incentive, which previous studies suggest is sufficient for actions that do not require individuals to change behavior on a sustained basis 31 . While having to pay a $20 co-pay for the vaccine did deter individuals, the additional economic incentives had no positive effect although they did not discourage vaccination 32 . Consistent with past research 31 , 33 , further analysis shows that the negative effect of the $20 co-pay was concentrated among low-income earners (Supplementary Fig. 7 ). Financial incentives failed to increase vaccination willingness across income levels.
Our study also yields important insights into the relationship between one prominent category of COVID-19 misinformation and vaccination preferences. We find that susceptibility to misinformation about COVID-19 treatments—based on whether individuals can distinguish between factual and false information about efforts to combat COVID-19—is considerable. A quarter of subjects scored no higher on our misinformation index than random guessing or uniform abstention/unsure responses (for the full distribution, see Supplementary Fig. 2 ). However, subjects who scored higher on our misinformation index did not exhibit greater vaccination hesitancy. These subjects actually were more likely to believe in vaccine safety more generally and to accept a COVID-19 vaccine, all else being equal. These results run counter to recent findings of public opinion in France where greater conspiracy beliefs were negatively correlated with willingness to vaccinate against COVID-19 34 and in Korea where greater misinformation exposure and belief were negatively correlated with taking preventative actions 35 . Nevertheless, the results are robust to alternate operationalizations of belief in misinformation (i.e., constructing the index only using false claims, or measuring misinformation beliefs as the number of false claims believed: see Supplementary Table 4 ).
We recommend further study to understand the observed positive relationship between beliefs in COVID-19 misinformation about fake treatments and willingness to receive the COVID-19 vaccine. To be clear, we do not posit a causal relationship between the two. Rather, we suspect that belief in misinformation may be correlated with an omitted factor related to concerns about contracting COVID-19. For example, those who believe COVID-19 misinformation may have a higher perception of risk of COVID-19, and therefore be more willing to take a vaccine, all else equal 36 . Additional analyses reported in the Supplementary Information (Supplementary Fig. 6 ) show that the negative effect of an EUA on willingness to vaccinate was concentrated among those who scored low on the misinformation index. An EUA had little effect on the vaccination preferences of subjects most susceptible to misinformation. This pattern is consistent with the possibility that these subjects were more concerned with the disease and therefore more likely to vaccinate, regardless of the process through which the vaccine was brought to market.
We also observe that skepticism toward vaccines in general does not correlate perfectly with skepticism toward the COVID-19 vaccine. Therefore, it is important not to conflate people who are wary of the COVID-19 vaccine and those who are anti-vaccination, as even medically informed individuals may be hesitant because of the speed at which the COVID-19 vaccine was developed. For example, older people are more likely to believe vaccines are safe but less willing to receive the COVID-19 vaccine in our survey, perhaps following the high rates of vaccine skepticism among medical staff expressing concerns regarding the safety of a rapidly-developed vaccine 2 . This inverse relationship between age and willingness to vaccinate is also surprising. Most opinion surveys find older adults are more likely to vaccinate than younger adults 37 . However, most of these survey questions ask about willingness to take a generic vaccine. Two prior studies, both recruiting subjects from the Lucid platform and employing conjoint experiments to examine the effects of vaccine attributes on public willingness to vaccinate, also find greater vaccine hesitancy among older Americans 6 , 7 . Future research could explore whether these divergent results are a product of the characteristics of the sample or of the methodological design in which subjects have much more information about the vaccines when indicating their vaccination preferences.
An important limitation of our study is that it necessarily offers a snapshot in time, specifically prior to both the election and vaccine roll-out. We recommend further study to understand more how vaccine perceptions evolve both in terms of the perceived political ownership of the vaccine—now that President Biden is in office—and as evidence has emerged from the millions of people who have been vaccinated. Similarly, researchers should consider analyzing vaccine preferences in the context of online vaccine controversies that have been framed in terms of patient autonomy and right to refuse 38 , 39 . Vaccination mandates may evoke feelings of powerlessness, which may be exacerbated by misinformation about the vaccines themselves. Further, researchers should more fully consider how individual attributes such as political ideology and race intersect with vaccine preferences. Our study registered increased vaccine hesitancy among Blacks, but did not find that skepticism was directly related to misinformation. Perceptions and realities of race-based maltreatment could also be moderating factors worth exploring in future analyses 40 , 41 .
Overall, we found that the most important factor influencing vaccine preferences is vaccine efficacy, consistent with a number of previous studies about attitudes toward a range of vaccines 6 , 42 , 43 . Other attributes offer potential cautionary flags and opportunities for public outreach. The prospect of a 50% likelihood of mild side effects, consistent with the evidence about current COVID-19 vaccines being employed, dampens likelihood of uptake. Public health officials should reinforce the relatively mild nature of the side effects—pain at the injection site and fatigue being the most common 44 —and especially the temporary nature of these effects to assuage public concerns. Additionally, in considering policy interventions, public health authorities should recognize that a $20 co-pay will likely discourage uptake while financial incentives are unlikely to have a significant positive effect. Lastly, belief in misinformation about COVID-19 does not appear to be a strong predictor of vaccine hesitancy; belief in misinformation and willingness to vaccinate were positively correlated in our data. Future research should explore the possibility that exposure to and belief in misinformation is correlated with other factors associated with vaccine preferences.
Survey sample and procedures
This study was approved by the Cornell Institutional Review Board for Human Participant Research (protocol ID 2004009569). We conducted the study on October 29–30, 2020, prior to vaccine approval, which means we captured sentiments prospectively rather than based on information emerging from an ongoing vaccination campaign. We recruited a sample of 1096 adult Americans via the Lucid platform, which uses quota sampling to produce samples matched to the demographics of the U.S. population on age, gender, ethnicity, and geographic region. Research has shown that experimental effects observed in Lucid samples largely mirror those found using probability-based samples 45 . Supplementary Table 1 presents the demographics of our sample and comparisons to both the U.S. Census American Community Survey and the demographics of prominent social science surveys.
After providing informed consent on the first screen of the online survey, participants turned to a choice-based conjoint experiment that varied five attributes of the COVID-19 vaccine. Conjoint analyses are often used in marketing to research how different aspects of a product or service affect consumer choice. We build on public health studies that have analyzed the influence of vaccine characteristics on uptake within the population 42 , 46 .
Conjoint experiment
We first designed a choice-based conjoint experiment that allowed us to evaluate the relative influence of a range of vaccine attributes on respondents’ vaccine preferences. We examined five attributes summarized in Table 2 . Past research has shown that the first two attributes, efficacy and the incidence of side effects, are significant drivers of public preferences on a range of vaccines 47 , 48 , 49 , including COVID-19 6 , 7 , 13 , 50 . In this study, we increased the expected incidence of minor side effects from previous research 6 to reflect emerging evidence from Phase III trials. The third attribute, whether the vaccine received full FDA approval or an EUA, examines whether the speed of the approval process affects public vaccination preferences 6 . The fourth attribute, the manufacturer of the vaccine, allows us to examine whether the highly public pause in the AstraZeneca trial following an adverse event, and the significant differences in brand familiarity between smaller and less broadly known companies like Moderna and household name Johnson & Johnson affects public willingness to vaccinate. The fifth attribute examines the influence of a policy tool—offsetting the costs of vaccination or even incentivizing it financially—on public willingness to vaccinate.
Attribute levels and attribute order were randomly assigned across participants. A sample choice set is presented in Supplementary Fig. 1 . After viewing each profile individually, subjects were asked: “If you had to choose, would you choose to get this vaccine, or would you choose not to be vaccinated?” Subjects then made a binary choice, responding either that they “would choose to get this vaccine” or that they “would choose not to be vaccinated.” This is the dependent variable for the regression analyses in Table 3 . After making a binary choice to take the vaccine or not be vaccinated, we also asked subjects “how likely or unlikely would you be to get the vaccine described above?” Subjects indicated their vaccination preference on a seven-point scale ranging from “extremely likely” to “extremely unlikely.” Additional analyses using this ordinal dependent variable reported in Supplementary Table 3 yield substantively similar results to those presented in Table 3 .
To determine the effect of each attribute-level on willingness to vaccinate, we followed Hainmueller, Hopkins, and Yamamoto and employed an ordinary least squares (OLS) regression with standard errors clustered on respondent to estimate the average marginal component effects (AMCEs) for each attribute 51 . The AMCE represents the average difference in a subject choosing a vaccine when comparing two different attribute values—for example, 50% efficacy vs. 90% efficacy—averaged across all possible combinations of the other vaccine attribute values. The AMCEs are nonparametrically identified under a modest set of assumptions, many of which (such as randomization of attribute levels) are guaranteed by design. Model 1 in Table 3 estimates the AMCEs for each attribute. These AMCEs are illustrated in Fig. 1 .
Analyzing additional correlates of vaccine acceptance
To explore the association between respondents’ embrace of misinformation about COVID-19 treatments and vaccination willingness, the survey included an additional question battery. To measure the extent of belief in COVID-19 misinformation, we constructed a list of both accurate and inaccurate headlines about the coronavirus. We focused on treatments, relying on the World Health Organization’s list of myths, such as “Hand dryers are effective in killing the new coronavirus” and true headlines such as “Avoiding shaking hands can help limit the spread of the new coronavirus 52 .” Complete wording for each claim is provided in Supplementary Appendix 1 . Individuals read three true headlines and five myths, and then responded whether they believed each headline was true or false, or whether they were unsure. We coded responses to each headline so that an incorrect accuracy assessment yielded a 1; a correct accuracy assessment a -1; and a response of unsure was coded as 0. From this, we created an additive index of belief in misinformation that ranged from -8 to 8. The distribution of the misinformation index is presented in Supplementary Fig. 2 . A possible limitation of this measure is that because the survey was conducted online, some individuals could have searched for the answers to the questions before responding. However, the median misinformation index score for subjects in the top quartile in terms of time spent taking the survey was identical to the median for all other respondents. This may suggest that systematic searching for correct answers is unlikely.
To ensure that any association observed between belief in misinformation and willingness to vaccinate is not an artifact of how we operationalized susceptibility to misinformation, we also constructed two alternate measures of belief in misinformation. These measures are described in detail in the Supplementary Information (see Supplementary Figs. 3 and 4 ). Additional regression analyses using these alternate measures of misinformation beliefs yield substantively similar results (see Supplementary Table 4 ). Additional analyses examining whether belief in misinformation moderates the effect of efficacy and an FDA EUA on vaccine acceptance are presented in Supplementary Fig. 6 .
Finally, model 2 of Table 3 includes a range of additional control variables. Following past research, it includes a number of demographic variables, including indicator variables identifying subjects who identify as Democrats or Republicans; an indicator variable identifying females; a continuous variable measuring age (alternate analyses employing a categorical variable yield substantively similar results); an eight-point measure of educational attainment; and indicator variables identifying subjects who self-identify as Black or Latinx. Following previous research 6 , the model also controlled for three additional factors often associated with willingness to vaccinate: an indicator variable identifying whether each subject had health insurance; a variable measuring past frequency of influenza vaccination on a four-point scale ranging from “never” to “every year”; beliefs about the general safety of vaccines measured on a four-point scale ranging from “not at all safe” to “extremely safe”; and a measure of attitudes toward the pharmaceutical industry ranging from “very positive” to “very negative.”
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data and statistical code to reproduce the tables and figures in the manuscript and Supplementary Information are published at the Harvard Dataverse via this link: 10.7910/DVN/ZYU6CO.
Hamel, L., Kirzinger, A., Munana, C. & Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020 | KFF (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
Article CAS PubMed Google Scholar
Scharf, I. & Ben Zion, I. As Vaccinations Lag, Israel Combats Online Misinformation. AP2 (21AD).
Nyhan, B. & Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33 , 459–464 (2015).
Article PubMed Google Scholar
Hussain, A., Ali, S., Ahmed, M. & Hussain, S. The anti-vaccination movement: a regression in modern medicine. Cureus 10 , e2919 (2018).
PubMed PubMed Central Google Scholar
Kreps, S. et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. open 3 , (2020).
Motta, M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med . 113642, https://doi.org/10.1016/j.socscimed.2020.113642 (2021).
Bokemper, S. E., Huber, G. A., Gerber, A. S., James, E. K. & Omer, S. B. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine , https://doi.org/10.1016/j.vaccine.2020.12.048 (2021).
Quinn, S. C., Jamison, A. M. & Freimuth, V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am. J. Public Health e1–e4 (2020).
Malik, A. A., McFadden, S. A. M., Elharake, J. & Omer, S. B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 26 , 100495 (2020).
Article PubMed PubMed Central Google Scholar
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Lazarus, J. V. et al . A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med . 1–4 (2020).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Heal ., https://doi.org/10.1016/s2468-2667(21)00012-8 (2021).
Wood, S. & Schulman, K. Beyond politics—promoting Covid-19 vaccination in the United States. N. Engl. J. Med . NEJMms2033790, https://doi.org/10.1056/NEJMms2033790 (2021).
Marshall, H. S., Chen, G., Clarke, M. & Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine 34 , 671–677 (2016).
Administration, F. and D. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry . (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med ., https://doi.org/10.1056/nejmoa2035389 (2020).
Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. (2020). Available at: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial . (Accessed 28 Feb 2021).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383 , 1920–1931 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383 , 2427–2438 (2020).
Quinn, S. C., Kumar, S., Freimuth, V. S., Kidwell, K. & Musa, D. Public willingness to take a vaccine or drug under emergency use authorization during the 2009 H1N1 pandemic. Biosecurity Bioterrorism 7 , 275–290 (2009).
Holden Thorp, H. A dangerous rush for vaccines. Science 369 , 885 (2020).
Limaye, R. J., Sauer, M. & Truelove, S. A. Politicizing public health: the powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother ., https://doi.org/10.1080/21645515.2020.1846400 (2020).
Feuer, W. Trump Says ‘No President’s Ever Pushed’ the FDA Like Jim, Vaccine Coming ‘Very Shortly’. CNBC (2020).
Soysal, A., Gokçe, I., Pehlivan, T. & Bakir, M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur. J. Pediatr. 166 , 533–539 (2007).
Greenberg, D. P. & Feldman, S. Vaccine interchangeability. Clin. Pediatrics 42 , 93–99 (2003).
Article Google Scholar
Bishai, D., Brice, R., Girod, I., Saleh, A. & Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. PharmacoEconomics 25 , 143–154 (2007).
Cameron, M. P., Newman, P. A., Roungprakhon, S. & Scarpa, R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 , 3712–3717 (2013).
Litan, R. Want herd immunity? Pay people to take the vaccine. Brookings (2020).
Kane, R. L., Johnson, P. E., Town, R. J. & Butler, M. A structured review of the effect of economic incentives on consumers’ preventive behavior. Am. J. Preventive Med. 27 , 327–352 (2004).
Volpp, K. G., Loewenstein, G. & Buttenheim, A. M. Behaviorally informed strategies for a National COVID-19 vaccine promotion program. JAMA - J. Am. Med. Assoc. 325 , 125–126 (2020).
Google Scholar
Nowalk, M. P. et al. Improving influenza vaccination rates in the workplace. A randomized trial. Am. J. Prev. Med. 38 , 237–246 (2010).
Bertin, P., Nera, K. & Delouvée, S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front. Psychol. 11 , 2471 (2020).
Lee, J. J. et al. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: cross-sectional online study. J. Med. Internet Res. 22 , e22205 (2020).
Reyna, V. F. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 30 , 3790–3797 (2012).
Lin, C., Tu, P. & Beitsch, L. M. Confidence and receptivity for covid‐19 vaccines: a rapid systematic review. Vaccines 9 , 1–32 (2021).
Broniatowski, D. A. et al. Facebook pages, the ‘Disneyland’ measles outbreak, and promotion of vaccine refusal as a civil right, 2009–2019. Am. J. Public Health 110 , S312–S318 (2020).
Moon, K., Riege, A., Gourdon-Kanhukamwe, A. & Vallée-Tourangeau, G. The Moderating Effect of Autonomy on Promotional Health Messages Encouraging Flu Vaccination Uptake Among Healthcare Professionals . (PsyArXiv, 2020), https://doi.org/10.31234/OSF.IO/AJV4Q .
Ferdinand, K. C., Nedunchezhian, S. & Reddy, T. K. The COVID-19 and influenza “Twindemic”: barriers to influenza vaccination and potential acceptance of SARS-CoV2 vaccination in African Americans. J. Natl. Med. Assoc. 112 , 681–687 (2020).
PubMed Google Scholar
Jaiswal, J., LoSchiavo, C. & Perlman, D. C. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 24 , 2776–2780 (2020).
Determann, D. et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE 9 , e102505 (2014).
Simpson, C. R., Ritchie, L. D., Robertson, C., Sheikh, A. & McMenamin, J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study. Lancet Infect. Dis. 12 , 696–702 (2012).
Remmel, A. COVID vaccines and safety: what the research says. Nature 590 , 538–540 (2021).
Coppock, A. & McClellan, O. A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Polit. 6 , 1–14 (2019).
de Bekker-Grob, E. W. et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine 36 , 1467–1476 (2018).
Determann, D. et al. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets–A discrete choice experiment in four European countries, 2013. Eurosurveillance 21 , 1–13 (2016).
de Bekker-Grob, E. W. et al. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 28 , 6692–6697 (2010).
Guo, N., Zhang, G., Zhu, D., Wang, J. & Shi, L. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine 35 , 2848–2854 (2017).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. USA 118 , 2021726118 (2021).
Hainmueller, J., Hopkins, D. J. & Yamamoto, T. Causal inference in conjoint analysis: Understanding multidimensional choices via stated preference experiments. Polit. Anal. 22 , 1–30 (2014).
Organization, W. H. Coronavirus disease (COVID-19) advice for the public: Mythbusters. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters . (Accessed 14 Jan 2021).
Download references
Acknowledgements
S.K. and D.K. would like to thank the Cornell Atkinson Center for Sustainability for financial support.
Author information
Authors and affiliations.
Department of Government, Cornell University, Ithaca, NY, USA
Sarah Kreps & Douglas L. Kriner
Injury Prevention Research Center, University of North Carolina, Chapel Hill, NC, USA
Nabarun Dasgupta
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
John S. Brownstein
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, USA
Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
Yulin Hswen
You can also search for this author in PubMed Google Scholar
Contributions
S.K. and D.K. designed the experiment/survey instrument and conducted the statistical analysis. S.K., N.D., J.B., Y.H., and D.K. all contributed to the conceptual design of the research and to the writing of the paper.
Corresponding author
Correspondence to Douglas L. Kriner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kreps, S., Dasgupta, N., Brownstein, J.S. et al. Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation. npj Vaccines 6 , 73 (2021). https://doi.org/10.1038/s41541-021-00335-2
Download citation
Received : 25 January 2021
Accepted : 06 April 2021
Published : 14 May 2021
DOI : https://doi.org/10.1038/s41541-021-00335-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Public perspectives on covid-19 public health and social measures in japan and the united kingdom: a qualitative study.
- Saki Kawamitsu
- Tin Zar Win
- Chris Smith
BMC Public Health (2024)
Public and health care provider attitudes, understanding, and behaviors regarding emergency use authorizations: a scoping literature review
- Caroline Rains
- Kathryn J. Aikin
- Sandra Crouse Quinn
Progress with COVID vaccine development and implementation
- Richard W. Titball
- David I. Bernstein
- Alan D. T. Barrett
npj Vaccines (2024)
COVID-19 vaccine refusal is driven by deliberate ignorance and cognitive distortions
- Kamil Fuławka
- Ralph Hertwig
- Thorsten Pachur
Knowledge, attitudes and demographic drivers for COVID-19 vaccine hesitancy in Malawi
- Yamikani Ndasauka
- Halima Sumayya Twabi
- Catherine Makhumula-Mtimuni
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

IMAGES
VIDEO