- Medical Services
- Schedule an Appointment
- Children's Health
- Hollings Cancer Center
- Education at MUSC
- Research at MUSC
- Clinical Trials

Clinical Trials & Research at MUSC
Research is essential to MUSC’s overall mission to preserve and optimize human life in South Carolina and beyond. Dedicated scientists and physicians work together through clinical research to improve treatments and advance our medical understanding. This research process is key to finding treatments and therapies that are safe and work for everyone.
A clinical trial is just one type of research study. In research studies, scientists and doctors gather information to answer questions and solve problems. There are different types of studies, such as clinical trials (which look at new treatments like drugs or devices), observational studies, and survey studies. Research studies are important because they help find better ways to prevent, screen for, diagnose, and treat diseases.
Kerry's Story
Clinical trials start with a group of people who have the same health condition, like a specific type of cancer or a certain disease. Each trial has a plan that explains what will be done, how it will be done, and why each part is necessary. There are also rules about who can join each study.
One big part of any research study is the group of people who volunteer. Some studies need volunteers who meet specific criteria. These volunteers may need to have a certain disease, be a certain age, gender, or ethnicity. Other studies may have fewer restrictions or be looking for healthy volunteers. The type of volunteer needed depends on the research question the study is trying to answer.
Frequently Asked Questions
Find answers to our most frequently asked questions about clinical trials.
People volunteer for a clinical trial for a variety of reasons, including the potential benefit for themselves or their loved ones, the opportunity to do something that may help others, and to join the scientific community in advancing healthcare. One of the easiest ways to understand why research (and, more importantly, volunteering for research) is important is to consider the fact that every medication that people take is available because of people who agree to participate in research studies. The decision to participate (or not participate) in a research study is voluntary. Those who decide to participate in a study are free to withdraw at any time without penalty.
Sometimes, people are hesitant to participate in research because they have concerns for their safety. It is important to note that every single research study is required to be reviewed for participant safety and protection. Safety is the number one priority for every study. In the United States, an independent committee of physicians, other scientists, and members of the community must approve and monitor the protocol for the safety of the participants. This committee is tasked with making sure that the potential risks of participating are reasonable in relation to the potential benefits the participants may gain. MUSC’s committee is called the Institutional Review Board (IRB).
The MUSC Hollings Cancer Center is one of 72 National Cancer Institute-designated cancer centers in the nation and the only one in South Carolina. This research that happens at the MUSC Hollings Cancer Center is the driving force behind delivering medical advances to patients and their families, enabling us to educate health care professionals and the public, and to establish outreach services for underserved populations across the state.
The following resources can help you find a study that may be right for you.
SCresearch.org - The South Carolina Research Studies Directory designed specifically to help people, like you, locate research studies in which to participate. Browse by topic, location, and more.
Researchmatch.org - A not-for-profit online resource that matches people who want to participate in research with researchers throughout the country who need volunteers for their studies.
Call 843-792-8300 and speak to a research professional about other study opportunities available at MUSC.
The Office for Human Research Protections (OHRP) Resources provides a variety of comprehensive resources about research participation and making informed decisions about research participation.
The National Institutes of Health (NIH) provides wide-ranging resources concerning the research process, adult and child participation in research, and national research opportunities.
MUSC provides the highest level of care to both current and future generations. Research is at the core of making this possible, and patients are the core of research. There are several ways patients at MUSC may hear about potential research studies. One of the most common ways is at a regular doctor’s visit, where a patient’s doctor may present information on a research study as a treatment option for an illness. Since MUSC is an academic medical center that places an emphasis on research, MUSC patients may be contacted directly by research personnel with information about research opportunities. Based upon a patient’s medical history, a study doctor (or someone working with a study doctor) may contact the patient if there is the possibility that the patient may meet the criteria for participation in a specific research study.
Just as research participation is voluntary, patients at MUSC have a choice in determining how they are informed of research opportunities. Patients who do not want to be directly contacted by MUSC research personnel about potential research opportunities may choose to “ opt-out .”
MUSC continuously tracks patient's research contact to prevent patient burden. You will also not be contacted about every study; only those that for which you may have interest based on your medical history or demographics. When a study team contacts you, it is only to provide you information and determine your level of interest in learning more about the study. Being contacted by research personnel does not mean that you are automatically part of a research study or that you are required to participate. Research participation is voluntary.
If you would like to opt out of being contacted directly for research participation opportunities, please complete the steps outlined here . Choosing to opt-out will in no way effect the quality of care you receive as an MUSC patient. Please note, however, that even if you choose not to be contacted directly by MUSC research personnel, your doctors may still suggest research studies that they feel may assist with your medical care. If you have decided to opt-out of receiving direct contact about potential research studies but change your mind in the future, you can return to your MyChart account at any time to update your preference .
Please call 843-792-8300 if you have questions.
MUSC Research Center – Columbia
MUSC Research Center – Florence
MUSC Research Center – Lancaster
Still have questions? Check out the FAQs here or reach out to research support staff at 843-792-8300 .

Coastal Carolina Research Center
Advancing medicine & improving lives, reliable clinical data, our mission, charleston's private medical research facility, our history, how we started and who we are, our proudest moments, historical milestones.

Need Help Finding a Study?

Innovative. Efficient. Reliable.
The Duke Clinical Research Institute, part of the Duke University School of Medicine, is the world’s largest academic clinical research organization. We conduct innovative research to deliver on our mission to share knowledge that improves health around the world. DCRI projects are led by renowned experts and physician scientists whose grounding in patient care helps to inform their research. Their work is supported by staff who have deep expertise in operationalizing global studies.
Stay informed of research news regarding our thought leaders, collaborations, and partnerships.

New Publication Investigates Practices to Improve Evidence Generation System in Clinical Trials
DCRI Shares Late-Breaking Results, Expert Insights at ESC Congress

First Parkinson’s Disease Patient Treated with ALX-001 in Allyx Therapeutics, DCRI Study

Expediting Evidence-Based Solutions for Patients across Diseases

Led by World-Renowned Experts

- PI Instructions
- About Clinical Trials
- About Pediatric Research
Through research, new discoveries are made possible

Search Studies:


M3 Wake Research Charleston
Clinical research studies focused on neurology, metabolic disease, pulmonology, and vaccines
2270 Ashley Crossing Drive, Suite 175 Charleston, SC 29414
MAIN OFFICE
843-258-1852
[email protected]
Mon-Fri 9-5
Your Local Healthcare Partner
M3 Wake Research Charleston is a distinguished site within the M3 Wake Research network, renowned for its commitment to excellence in conducting a wide range of clinical studies in the Southeast.
Currently Enrolling Studies & Specialties in the Charleston, South Carolina, Area
- Chronic Obstructive Pulmonary Disease (COPD) →
- Hypertension →
- Severe Asthma →
- Severe Hypertriglyceridemia →
- Vaginal Atrophy/Vaginal Dryness →
- General Interest →
SPECIALTIES
- Flu, COVID, RSV →
- Pulmonary →
- Cardiology →
- Vaccines →
- Women’s Health →
What Participants Are Saying

The staff have been exceptional, every step was organized, and professional, full explanation of each procedure. And kept me well-informed. I had such an exceptional experience; I look forward to being part of a future study. Many thanks to all. — Kathy Forest

M3 Wake Research was always efficient. My appointments were always on time. The visits and calls were thorough. Being able to call anytime with questions and concerns made my participation easy. I look forward to more studies in the future. — Teresa Starks

M3 Wake Research was very efficient, friendly, and professional. I was happy to be involved in this clinical study. I encourage anyone interested in clinical studies to give them a try. It was a great experience, plus I got the added benefits of receiving the vaccine. — Diane Matranga

They were all very great, professional, courteous, nice, and very friendly. They treated me awesome and took care of me very efficiently. — Harold Kessee

The staff was very accommodating with scheduling. I appreciate their flexibility with changes to my appointments. Also, I never got bruised from blood draws. The clinical staff is experienced and thoughtful. I feel well taken care of. Thank you! — Patrick Yeung
Meet the Principal Investigator
Donald hurley, do .
Board-certified and a Fellow with the American Academy of Family Physicians
Dr. Don Hurley is trained in the full complement of the specialty, including traditional care and innovative techniques. He is one of two Certified Physician Investigators in South Carolina, and rated one of the Best Physicians in the country three times. He was chosen to be the Primary Care Physician for the White House in Savannah, Georgia, at the GB conference, caring for thousands of international diplomats. Dr. Hurley chose Osteopathic Medicine because it is founded in the belief that the physician should treat the whole person rather than just the symptoms.
Amelia Fairfax, MD
Fourteen years of practicing in South Carolina and the Lowcountry
Practicing Family Medicine in the Lowcountry has been a great fit for Dr. Fairfax’s passion for preventative medicine and primary care.

Meet The Team
Vanessa Armetta Lead Clinical Research Coordinator 7+ years of experience in the research industry
Vanessa manages Phase I – IV studies in the areas of cardiovascular, endocrinology, women’s health, dermatology, and various vaccine and pharmaceutical clinical trials.
Partner with M3 Wake Research in Charleston, SC
High standards of trial conduct & patient care.
M3 Wake Research Charleston, as part of the M3 Wake Research network, specializes in conducting clinical trials with a focus on patient safety and scientific integrity. Our collaboration ensures access to a range of therapeutic areas, maintaining the high standards of trial conduct and patient care through our network’s robust quality assurance and protocol standardization.
Cutting edge of medical research
By partnering with M3 Wake Research Charleston, healthcare providers can offer their patients access to pioneering treatments across various medical disciplines. Our clinical trials in the Lowcountry represent the cutting edge of medical research, providing new avenues for patient care. This partnership benefits patient health outcomes, and underscores your practice’s dedication to innovation and quality care.
The Wake Difference
We are committed to advancing the treatment landscape through seamless clinical trials, patient access with business associate agreement (baa).
- BAA with fee arrangement for patient access
- Collaboration in patient outreach
Patient Access with BAA+ Onsite Advertising
- Additional fee for onsite advertising to promote clinical trials to patients
- Regular site staff visits for enhanced collaboration
Friendly Referral Relationships
- Informal, no-payment agreement
- Education and materials provided by our staff
- Regular engagement sessions to maintain a collaborative relationship
News & Updates

Understanding Behavioral Health Disorders
September 7, 2024
Understanding Gastrointestinal Diseases: An Overview of GI Disorders
September 5, 2024

The Importance of Women’s Health
September 3, 2024

Defining Epilepsy, Its Causes, and How It Affects the Brain
August 23, 2024

Join or Conduct Research Studies
Contact m3 wake research charleston in charleston, south carolina.
Whether you are a patient, caregiver, or a physician, clinical trials are available in the Charleston, South Carolina, area for a variety of focus areas. Our team at M3 Wake Research Charleston provides the highest quality research studies to advance the medical field and find innovative treatments and therapies.
Use our Find a Trial tool or search by indication and your location to find a clinical trial that may be relevant to your condition. If you need assistance or have any questions, please contact us directly .

An integrated research organization committed to revolutionizing clinical research and expanding access to clinical trials.
- Social via Facebook
- Social via Twitter
- Social via Linkedin
How Clinical Trials Work
Clinical Trials FAQ
Our Clinics
Partner With Us
© 2024 All Rights Reserved
- Privacy Policy
- Terms of Use
- Coronavirus Updates
- Education at MUSC
- Adult Patient Care
- Hollings Cancer Center
- Children's Health
Office of the Vice President for Research
Fostering curiosity and compassion to perform transformative academic research to improve the quality of life for all, from bench science to translational research.
Research at MUSC
Innovating transformative care.
We’re harnessing the possibilities of innovation. At the Medical University of South Carolina, new ideas and advancements flow from classrooms to laboratories and out into communities across our state and the world. Led by our internationally recognized faculty and scientists, our discoveries grow knowledge, our clinical trials transform medical treatments, and our innovations influence the care you receive.
As South Carolina’s premier biomedical research institute, we know there’s always new terrain to cover. The drive toward innovation that makes an impact propels us forward: leading the state in research funding, we’re home to just one of 60 Clinical and Translational Service Award (CTSA) hubs, our researchers published over 3,500 articles in 2021 alone, and we’re the home of more than 50 active biomedical startups. See how we’re shaping the boundless discoveries of research.
Featured Research Stories

Medulloblastoma Research
The Group 3 subtype of medulloblastoma has the worst prognosis of all the subtypes, but new research is pointing to a potential therapy.

Dangerous Associations
MUSC scientists identify how cocaine triggers a specific protein in the brain to hijack normal brain circuitry and promote further drug taking.
Hooked on a Feeling
Opioid receptors in a new brain region, the dorsal peduncular nucleus, act in a unique way to elicit opioids’ rewarding effects.

A new study aims to get a better understanding of what dual use of vapes and cigarettes looks like in real world usage.

Depression and Cancer
Two Hollings researchers are working together to develop a new way to get depression treatment to people with likely incurable cancer.
Glioma Drug Approval
Hollings was part of an international clinical trial that led to the FDA approval of a new drug to treat certain types of gliomas.
Read more articles from the MUSC News Centers about exciting research happenings.
Research Areas
Innovation lays the foundation for advancement, but it doesn't stop there. Explore how our discoveries impact the lives we touch–from new diagnostic approaches to more elective, less invasive treatments.

Addiction Sciences
From brain neuroimaging to genetics, researchers use technology to increase the medical field's understanding of addiction and expand treatment possibilities.
We're home to Hollings Cancer Center, the state's only NCI-designated center that serves as a hub of innovative research, education, treatment, and outreach.
Community Health
Drawing together community members and researchers, we strive to understand and break down health disparities and improve healthcare access across our state.
Digestive Diseases
As an emerging leader in this area, MUSC connects gastroenterologists, surgeons, and radiologists to advance treatment of digestive health and liver disease.
Drug Discovery
Our collaborations with pharmaceutical and biotechnology industries generate breakthroughs for fibrotic and inflammatory diseases and neurological disorders.

Health Disparities
We aim to increase healthcare access and reduce care disparities in the state while focusing on conditions ranging from cancer and diabetes to mental illness.
Inflammation Fibrosis
Our preeminent basic, clinical, and translational research advances therapies for autoimmune disorders and fibrotic diseases.
Neuroscience
MUSC has one of the nation's largest teams of neuroscience specialists using the latest technologies to learn how the brain works and advance the field.
Oral Health
Our use of technology paired with state-of-the-art dental education leads to discoveries that improve oral, dental, and craniofacial health.
Stroke & Spinal Cord Injury
Our advances in stroke and spinal cord injury research shape approaches to post-stroke and rehabilitation care.
Centers, Cores & Institutes
Transformative ideas begin here. Explore MUSC’s research centers, institutions, and cores, home to state-of-the-art resources and health-advancing instruction.

Centers and Programs

Research Cores

Find an Expert
MUSC recruits top scientists to push boundaries and direct innovation. Get to know our world-recognized team.

Find a Clinical Trial
Empowering patient health begins with clinical trials. Explore how we’re pioneering progress.

Resource Routing
Innovation flourishes through collaboration. Learn more about our health-advancing startups and funding opportunities, and help us continue unlocking transformative possibilities.
Research Development
Identifies extramural funding opportunities and assists faculty members through grant proposal development and by provides strategic educational resources.

Partnerships
Works with industry sponsors, contract research organizations, and other research collaborators to pair clinical research opportunities with MUSC Investigators.

Research & Sponsored Programs
Aids in managing and overseeing extramurally-funded programs, ensuring compliance and serving as the authorized point of contact for sponsored projects.

Funding Portal
Enables MUSC Investigators to find federal, state, MUSC Foundation, corporate, and pilot funding opportunities to support the various stages of their research.

Tools for Researchers
Access a range of resources for MUSC researchers, including cores, funding information, post-award management, IP development, sustainability tips, and more.
SCTR Institute
Improves health outcomes and quality of life for South Carolina and beyond through discoveries translated into evidence-based practice.

Velocity is the world's leading integrated site organization.
Sponsors and cros trust velocity to deliver high-quality clinical trial data and patient care with unprecedented efficiency., simplify everything from site selection to study close-out.
Velocity unifies operational processes to provide world-class sites, reliable enrollment, and predictably high performance for your trials.
The right sites. The right investigators. The right partner for you.
Strategically located to give you access to diverse specialty populations, Velocity's sites are supported by next-gen technologies and patient engagement capabilities. Welcome to recruitment and retention reimagined.
Scale for a purpose: Supporting research programs worldwide
From the leading pharma companies, to the most pioneering biotech startups, Velocity supports those who are exploring new frontiers in human health.
Whether you’re ready to conduct a single-site study or a complex, high-volume clinical trial, contact Velocity.

Velocity Has Supported Research for 12 Products That Have Received FDA Approval in 2024
Velocity sites and investigators have now supported research for 12 products that received U.S. FDA approval in 2024! This year’s groundbreaking approvals include: Ebglyss (lebrikizumab-lbkz) by Eli Lilly, a new … Read more

Raghu Punnamraju, CTO, for VentureBeat: Why data science alone won’t make your product successful
In this new article for VentureBeat, Raghu Punnamraju, Chief Technology Officer at Velocity, discusses why data science alone is not enough to ensure product success. In this insightful piece, Raghu … Read more

Paul Evans Featured in Business North Carolina Article on AI in the State’s Clinical Research Industry
Paul Evans was recently featured in a Business North Carolina article discussing the emerging impact of artificial intelligence on North Carolina’s clinical research industry. As Paul notes, “Our industry is … Read more

Drs. Essink and Overcash Author Article Featured in Nature Communications for Moderna COVE Study
Brandon Essink, MD, CPI, and J. Scott Overcash, MD, were authors of a recent Nature Communications article, “Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and … Read more
Quality. Continuity. Velocity.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Cookie Policy (US) Cookie Policy (EU) Cookie Policy (UK)

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Decentralized Clinical Trials for Medications To Reduce the Risk of Dementia: Consensus Report and Guidance
This article describes recommendations for the conduct of remote medication trials for dementia prevention. The recommendations were crafted by an expert panel and refined by a working group of dementia researchers and clinicians. The article contains 40 recommendations for this trial type, including guidelines for safety, dispensing, outcome assessment, and data collection.
Howard L, et al. Decentralized clinical trials for medications to reduce the risk of dementia: Consensus report and guidance . Alzheimer’s & Dementia . 2024;20(7):4625-4634. doi: 10.1002/alz.13891.
Disclaimer: The inclusion of a resource in ADORE does not represent an endorsement by NIA or the broader National Institutes of Health and U.S. Department of Health and Human Services.
nia.nih.gov
An official website of the National Institutes of Health
- Grants & Funding Opportunities
- Study Design, Data & Analysis
- Conducting Clinical Research
- Patient/Community Engagement
- Training & Career Development
- Implementation Science
- Commercialization
- Recruitment & Retention
- Research Space
- News & Announcements
- Policies & Regulations
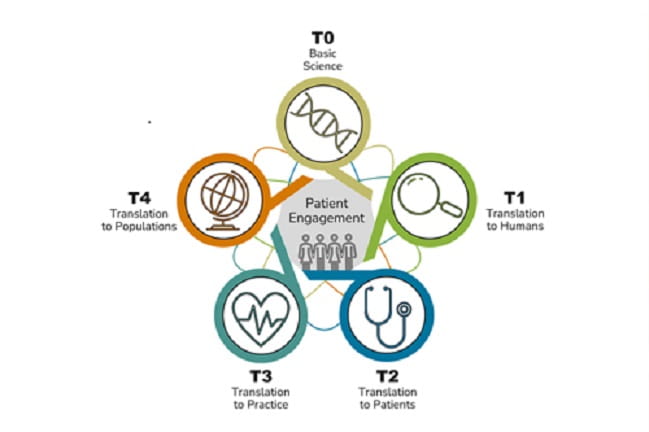
- Our Mission and Partners
- What is Translational Science?
- Leadership Team
- Use of services policy
- Cite the grant
- Contact / Directions
UNC Chapel-Hill:
- Forgot your password?
- Forgot your username?
- All News Articles
Call for Nominations: 2025 Top 10 Clinical Research Achievement Awards
- 16 September 2024
Studies published in a peer-reviewed journal during calendar year 2024 are eligible

The Clinical Research Forum has opened the call for nominations for the Annual Top 10 Clinical Research Achievement Awards.
The awards recognize outstanding achievements in clinical research from across the nation and honor major advances in the biomedical field resulting from the nation's investment in health and welfare.
Nominations for outstanding research projects should:
- Represent innovation, creativity and scientific advancement
- Contribute to the understanding of human disease and/or human physiology
- Demonstrate impact on the prevention, diagnosis and/or treatment or increased understanding of the disease state
Three of the studies will receive additional recognition and cash awards.
- Herbert Pardes Clinical Research Excellence Award — $7,500 is awarded to the one study that best exemplifies a high degree of innovation and creativity, advances science, and has an impact upon human disease.
- Distinguished Clinical Research Achievement Award — $5,000 is awarded to two studies that show creativity, innovation, or a novel approach that demonstrates an immediate impact on the health and well-being of patients.
All Top 10 winners will also be interviewed for the Journal of Clinical and Translational Science .
For more information, and to submit a nomination, visit www.clinicalresearchforum.org .
Submissions due October 11, 2024 .
Research studies eligible for nomination must have been conducted at a U.S. institution and published in a peer-reviewed journal between January 1, 2024, and December 31, 2024. A letter or e-mail from a peer-reviewed journal confirming a publication date prior to January 1 is acceptable.
View news related to policies and regulations Have news or an announcement to share? Contact Michelle Maclay at [email protected] Get NC TraCS events and news delivered to your inbox! Subscribe to our weekly email blast
In partnership with:.

Brinkhous-Bullitt, 2 nd floor 160 N. Medical Drive Chapel Hill, NC 27599

© 2008-2024 The North Carolina Translational and Clinical Sciences (NC TraCS) Institute at The University of North Carolina at Chapel Hill The content of this website is solely the responsibility of the University of North Carolina at Chapel Hill and does not necessarily represent the official views of the NIH accessibility | contact

- Study Abroad Get upto 50% discount on Visa Fees
- Top Universities & Colleges
- Abroad Exams
- Top Courses
- Read College Reviews
- Admission Alerts 2024
- Education Loan
- Institute (Counselling, Coaching and More)
- Ask a Question
- College Predictor
- Test Series
- Practice Questions
Course Finder
- Scholarship
- All Courses
- B.Sc (Nursing)
Noida Phistar Clinical Res...

Phistar Clinical Research Institute, Noida

Phistar Clinical Research Institute Fees & Eligibility
| Course | Fees | Eligibility | Action |
|---|---|---|---|
| 2 | ₹ | 10+2 |
Do you think the Course details are wrong ? Report Here
Courses Offered By Phistar Clinical Research Institute 2024
Select Degree and Streams to See Course Fees and Admission Details.
Eligibility:
Do you think the fees are wrong ? Report Here
Search from 20K+ Courses and 35+ Streams
Popular Streams:
Popular courses:, phistar clinical research institute facilities.
You can check here to see what types of facilities provides.
Phistar Clinical Research Institute College Details
Basic Information About the Colleges
Discover More Colleges
![clinical research institute s.c Nightingale Institute of Nursing - [NIN]](https://image-static.collegedunia.com/public/college_data/images/appImage/12059_NINNOIDA_APP.jpg?h=111.44&w=263&mode=stretch)
Are You Interested in this College?

SUBSCRIBE TO OUR NEWS LETTER

- Equality, Diversity and Inclusion
- Strategy and Funding
- Influencing Policy
- Research Policies
- Branding and Logos
- Admin Groups
- Campus Connections
- Collaborations
- Sanger Projects
- Publications
- Sanger Seminar Series
- Associate Faculty
- Honorary Faculty
- International Fellows
- Science Staff
- Non-science Staff
- Innovation at the Institute
- For Industry
- For Researchers
- Case Studies
- Our Spin-Outs
- Sanger Technologies
Breast and ovarian cancer newly linked to thousands of gene variants
Email newsletter.
News and blog updates
Listen to “Breast and ovarian cancer cancer newly linked to thousands of gene variants” on Spreaker.
Scientists have pinpointed thousands of genetic changes in a gene that may increase a person’s risk of developing breast and ovarian cancer, paving the way for better risk assessment and more personalised care.
Researchers from the Wellcome Sanger Institute and their collaborators focused on the ‘cancer protection’ gene RAD51C , known to increase ovarian cancer risk six-fold and risk of aggressive subtypes of breast cancer four-fold 1 . They found over 3,000 harmful genetic changes that could potentially disrupt its function and increase cancer risk. These findings were confirmed by analysing data from large-scale health databases.
The findings, published today (18 September) in Cell , are freely available so that they can be immediately used to help doctors and diagnostic laboratory scientists better assess cancer risk, especially for individuals with a family history of these cancers, reducing the uncertainty that often accompanies genetic testing.
The study also identified regions of the protein essential for its function, pointing to new roles in cancer development and potential therapeutic targets.
Breast cancer is the most common cancer in the UK, with around 56,800 new cases every year. One in seven UK females will be diagnosed with breast cancer in their lifetime 2 . Ovarian cancer is the sixth most common cancer in females in the UK, with around 7,500 new cases every year 3 .
The RAD51C gene encodes a protein crucial for DNA repair. Variants in this gene that stop the protein from working are known to increase the risk of breast and ovarian cancers and rarely, if there are two harmful gene changes are present, may result in Fanconi Anaemia, a severe genetic disorder 4 . Women with a faulty RAD51C gene face a 15 to 30 per cent lifetime risk of developing breast cancer and a 10 to 15 per cent risk of developing ovarian cancer 5 .
While genetic testing is common for individuals with a strong family history of cancer, the health impacts of most RAD51C variants were previously unknown. This uncertainty over cancer risk often leaves patients and doctors struggling to determine appropriate medical care moving forward.
In this new study, researchers from the Wellcome Sanger Institute and their collaborators set out to understand the effect of 9,188 unique changes in the RAD51C gene by artificially altering the genetic code of human cells grown in a dish, in a process known as ‘saturation genome editing’. They identified 3,094 of these variants that may disrupt the gene’s function and increase cancer risk, with an accuracy above 99.9 per cent when compared to clinical data. Analysis of UK Biobank data and an ovarian cancer cohort of over 8,000 individuals further confirmed the link between these harmful RAD51C variants and cancer diagnoses.
By mapping the protein structure, the team also identified crucial surface areas of RAD51C essential for its DNA repair function. These regions may interact with other, yet-to-be-identified proteins or play a role in processes such as phosphorylation, offering valuable insights for drug development and potential new treatment targets.
The study also revealed the existence of ‘hypomorphic alleles’ – a type of variant that reduces the RAD51C gene’s function without completely disabling it. These appear to be more common than previously thought and may significantly contribute to breast and ovarian cancer risk.
“This research demonstrates that genetic risk for breast and ovarian cancer isn’t a simple yes-or-no scenario, but exists on a spectrum based on how genetic changes affect protein function. With a more comprehensive understanding of how RAD51C genetic variants contribute to cancer risk, this opens up new possibilities for more accurate risk prediction, prevention strategies, and potentially targeted therapies.” Rebeca Olvera-León, first author of the study at the Wellcome Sanger Institute
“This work demonstrates the power of analysing genetic variants on a large scale within their genomic context. Not only can we understand how cancer-related DNA changes affect patients, helping with clinical decisions, but we can also explore how these variants impact the gene’s function at a detailed molecular level. This provides important insights into how proteins work and how genes evolve over time.” Dr Andrew Waters , co-senior author of the study at the Wellcome Sanger Institute
“The strong connection between harmful variants and cancer in large studies suggests that this approach to variant classification could be a valuable tool in personalised medicine and cancer prevention. We aim to extend this technique to many other genes, with the goal of covering the entire human genome in the next decade through the Atlas of Variant Effects.” Dr David Adams , co-senior author of the study at the Wellcome Sanger Institute
“These new data will be highly useful for diagnostic laboratories to better understand the RAD51C gene changes that we identify on clinical genetic testing in cancer patients and their family members. The assay data will help us to conclude which gene changes are harmful and which are innocent. This aids our decision making regarding which patients might benefit from offer of extra breast cancer screening and preventive surgery of the ovaries.” Professor Clare Turnbull, clinical lead of the study, Professor of Translational Cancer Genetics at The Institute of Cancer Research, London, and Consultant in Clinical Cancer Genetics at The Royal Marsden NHS Foundation
More information
References:
- Hu et al. (2024) ‘Functional and Clinical Characterization of Variants of Uncertain Significance Identifies a Hotspot for Inactivating Missense Variants in RAD51C ’ Cancer Research . DOI: 10.1158/0008-5472.CAN-22-2319
- https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer
- https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer
- Anaemia Fanconi anaemia is rare and occurs in 1 in 160,000 individuals worldwide, and can happen when a child inherits a faulty RAD51C variant from both parents. 17 other genes have been causally associated with the development of Anaemia Fanconi. https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/fanconi-anaemia/
- https://www.facingourrisk.org/info/hereditary-cancer-and-genetic-testing/hereditary-cancer-genes-and-risk/genes-by-name/rad51c/cancer-risk#:~:text=Women%20with%20a%20RAD51C%20mutation%20have%20about%20a%2010%2D15,to%20together%20as%20ovarian%20cancer ).
Publication: R. Olvera-León et al. (2024) ‘High-resolution functional mapping of RAD51C by saturation genome editing.’ Cell . DOI: 10.1016/j.cell.2024.08.039
Funding: This research was supported by Wellcome and Cancer Research UK. For full funding acknowledgements, please refer to the publication.
Quick links
Dave Adams' profile
Adams research group
News story: Map of disease-causing mutations in neurodevelopmental disorders and cancer revealed
News story: Thousands of high-risk cancer gene variants identified
Latest news
18 Sep 2024
New research identifies specific genetic changes that can increase a person’s risk of breast and ovarian cancers, to help guide ...

11 Sep 2024
New genetic links between menopause and cancer risk
Researchers have found multiple genes linked to the timing of menopause and provide new insight into links with cancer risk.

Common diabetes drug slows growth of normal cells carrying cancer mutation
Our tissues are colonised by mutant cells as we age. New research finds metabolic health and antidiabetic drugs affect the growth ...
Office of the Vice President for Research
UI launches Implementation Science Center to translate research into practice
A new Board of Regents-approved University of Iowa Implementation Science Center (ISC) will help ensure that research findings are translated into real-world practices that benefit people as quickly and efficiently as possible.
“Unfortunately, on average, it takes 17 years for research to be incorporated into routine practice in hospital or community settings,” said Heather Schacht Reisinger, professor of internal medicine in the Carver College of Medicine, and director of the new center. “The emerging field of implementation science seeks to close that gap.”
Implementation science explores the organizational and behavioral factors that are important for ensuring that evidence-based practices reach all who could benefit as quickly as possible. The process involves engagement with key partners and communities to identify the best methods for integrating research findings in a way that impacts the overall health of a community.
“As scientists, we are trained to communicate to other scientists through publications or conferences,” said Reisinger. “Implementation sciences takes this work a step further, ensuring that it doesn’t stay on the shelf. By also studying people, organizations, and systems, we can more effectively and efficiently help them integrate evidence-based practices into what they are already doing.”
Building on the UI's strengths

Reisinger is a medical anthropologist with a depth of experience in the field of implementation science. She was a co-author of National Cancer Institute’s Qualitative Methods in Implementation Science white paper; is an Associate Editor for Implementation Science , the international flagship journal in the field; and presented at China’s first implementation science conference.
The new center, which reports to the Office of the Vice President for Research with support from the Provost Office, Carver College of Medicine, Holden Comprehensive Cancer Center, College of Public Health, and College of Nursing, will serve as convening hub for scientists and practitioners from the health science colleges and beyond.
“I’m excited to leverage my expertise and work with my colleagues across campus to build our capacity to conduct implementation science and contribute to a growing body of knowledge about what strategies and tools are most effective at moving research into practice,” said Reisinger. In the coming months, Reisinger will assemble an advisory committee to help shape the center’s structure and strategic plan. The ultimate goal is to create new paradigms in the field and incorporate implementation science into researchers’ existing projects.
“This work closely aligns with the goals outlined in the University of Iowa Strategic Plan , including to expand the university’s impact on local and regional communities, the state of Iowa, and the world by leveraging transformational research and discovery,” said Lois Geist, interim vice president for research and associate provost for faculty.
Training in the application of evidence-based practices is not new to the UI. The College of Nursing has a 30-year tradition of mentoring and training nurses in evidence-based practice through the Iowa Model . “We have the opportunity to learn from and build on this model within the field of implementation science to create a pathway for practitioners across healthcare,” said Reisinger.
The UI is the only site in the state with a College of Public Health and a comprehensive basic-to-clinical research enterprise situated within an academic medical center. The new Implementation Science Center will establish and expand partnerships with a number of institutional units, including the Institute for Clinical and Translational Science, which works with Professor Reisinger to successfully integrate implementation science into their programs. She serves as the unit’s associate director of engagement, integration, and implementation.
Rural connections
Supporting the integration of evidence-based practice in rural communities is a key area of focus for the center. While implementation science is rapidly expanding, less attention has been paid to the uptake of evidence-based practices and the latest research in rural health settings.
“Iowa is well-situated to contribute to expanding implementation science into rural health due to our geographic location and the rural focus of many of the centers and institutes on campus, such as the Prevention Research Center for Rural Health and Rural Policy Research Institute,” said Reisinger.
The ISC is already working with the P3-funded project “ Reducing the Impact of Lung Cancer among Iowans through Prevention and Early Detection ,” to explore questions of rural implementation. The project involves faculty from College of Public Health and Carver College of Medicine. “We are reaching out to counties with higher lung cancer rates to work with healthcare systems to support implementation of successful lung cancer screening, as well as increasing access to radon testing and reviewing local tobacco retailer marketing for points of intervention,” said Reisinger. “Our goal is to have local impact in Iowa, while improving our understanding of implementation strategies that are most effective in rural settings.”
Get involved
Researchers who are interested in implementation science are invited to utilize the center’s available collaboration space and quiet workspaces on the second floor of the Medical Research Facility. “Although we have a physical location in the MRF, we envision moving around campus to host trainings, lectures, and networking events in an effort to meet researchers where they are,” said Reisinger.

On October 3 at 2:00 p.m. in the Nursing Clinical Education Center (W417 General Hospital), the center will host its first lecture of the semester, “ Building a Big Tent for Implementation Science Together: Reflections and Opportunities ” by David Chambers , deputy director for implementation science, Division of Cancer Control and Population Sciences, National Cancer Institute. After his lecture, Dr. Chambers will spend time with attendees who are interested in implementation science in cancer prevention and treatment to discuss funding opportunities at NCI. Virtual attendance will be available.
An implementation science journal club, where researchers discuss the latest research and their own works-in-progress, will be held on the third Thursday of the month at 1:00 pm in the Institute for Clinical and Translational Science (C44-A General Hospital). The journal club is held as a hybrid meeting. Additional details about the center’s activities will be posted at isc.research.uiowa.edu . Join the listserv and monthly newsletter by contacting [email protected] .
Photo credit: Acacia Lab, Southern Medical University, Ghangzhou, China
- Tools & Services
- OpenRegSource Regulatory Portal
- Funding Opportunities
- Proposal & Grant Writing Tips
- Previous Awardees
- For Research Professionals
- Mentored Career Development in Clinical and Translational Science Program (KL2)
- Educational Offerings
- Online Educational Library
- Education Resource Center (ERC)
- Healthcare Delivery Science
- Community Mentorship Program
- Individual Development Plans
- Research in Diverse Populations Curriculum
- Training // Digital Scholar Program
- Team Science Training
Get early access to treatment options and help advance research for cancer and other diseases.
- Education Offerings for Community Members
- Careers in Clinical and Translational Research
Tools & services for conducting research
Get education and training, obtain pilot funding, learn about team science, use informatics-based research tools and services, conduct community-engaged research, access outpatient clinical trials unit, learn about healthcare delivery science, hire a clinical research coordinator, receive guidance on regulatory processes, get help with biostatistics and research design, data cleaning and management.
Assisting investigators with preparing data for statistical analyses
Data Collection and Management Plans
Providing assistance to investigators with developing a data collection strategy
Data Sharing Plans
Providing guidance to investigators with specification and implementation of plans for sharing data
Research Electronic Data Capture (REDCap)
REDCap is a free, secure, web-based application designed for rapidly developing databases and surveys for research studies.
Scientific Writing Support
Providing assistance to investigators with writing grant sections and publications
Statistical Analysis
Assistance for investigators with the conduct, interpretation and summary of statistical analyses
Statistical Analysis Plans
Quality assistance for investigators with developing statistical analysis plans
Statistical Reports
Providing assistance to investigators with developing interim and final reports
Study Design
Professional assistance for investigators with a range of study design issues
USC Clinical Studies Directory
Helping USC researchers to connect with prospective clinical study participants
Academic or Community Partnership Assistance
Helping researchers identify partners and foster relationships across academic and community settings
Access Los Angeles Countywide Patient Data
LADR is an integration of medical records and clinical research data across all participating networks. Investigators are able to find sets of aggregated patient information through a web-based application all while preserving patient privacy.
Autism Promotor Manual
This manual was developed with the intent to address access the disparities and access to care for Autism Spectrum Disorder (ASD) and other developmental delays among Latino children in Los Angeles, California
Automated Assistance with Study Recruitment on Social Media
Use Trial Promoter to make your social media recruitment quicker, more streamlined and efficient.
NIH Funding Acknowledgment: Important - All publications resulting from the utilization of SC CTSI resources are required to credit the SC CTSI grant by including the NIH funding acknowledgment and must comply with the NIH Public Access Policy.

COMMENTS
SCTR is a statewide, NIH-funded institute that provides consultative expertise, resources, training, and funding to support research teams. Our goal is to accelerate scientific discoveries from the laboratory to clinical and public health practice to improve the health of patients and communities across SC and beyond. We Serve . Connect with Us
The Research Design, Compliance, and Data Management (RDCD) Core provides the infrastructure and expertise to enhance research compliance, provide biostatistics and research design support, and evaluate healthcare outcomes using advanced clinical design, electronic health records, data science techniques, and artificial intelligence.
The South Carolina Clinical & Translational Research Institute (SCTR) is part of the National Institutes of Health ... Charleston, SC 29425. Directions. View Details. SCTR Institute. Roper MOB. 125 Doughty Street. Suite: 140. MSC Code: 195. Charleston, SC 29403. Directions. Main: 843-792-8300.
About. As South Carolina's premier biomedical research institution, the Medical University of South Carolina (MUSC) is committed to recruiting top scientists to serve the needs of residents. With $300+ million in research funding and 1,242 extramural awards in fiscal year 2023, the collaboration between faculty, staff, students, and our ...
CCRC is a medical research facility focused on conducting clinical trials. We provide access for thousands of Charleston Lowcountry citizens who are interested in participating in clinical trials and research studies. ... CCRC recognizes that our most compelling opportunity is to replace the public's apprehension or misconception of clinical ...
If you have any questions regarding South Carolina Research Studies Directory, do not hesitate to contact us. Our office is open Monday through Friday, 8:30 am to 5:00 pm. South Carolina Clinical and Translational Research Institute. 125 Doughty Street, Suite 100, MSC 195. Charleston, SC 29425. Phone: 843-792-8300. Email: [email protected].
Check out the FAQs here or reach out to research support staff at 843-792-8300. General MUSC Health Line 843-792-1414. Information about clinical trials at MUSC Health and the State of South Carolina.
In addition, the SC CTSI provides free research navigation services to all faculty, staff, and students. If you have any questions, contact Clinical Research Support at 323-442-CTSA or [email protected]. Finally, this document is dynamic and meant to be updated regularly as processes change and new information becomes available.
Clinical Research Informatics (CRI) fosters efficient, secure and high-quality data access, management and interoperability. They are leading the implementation of our research data warehouse and facilitating the integration with the Clinical Trials Management System to compliment a full suite of electronic tools for research and the electronic ...
Coastal Carolina Research Center (CCRC) is a private, locally-owned medical research facility located in North Charleston, SC. CCRC is a "dedicated" medical research facility which means we are totally focused on conducting clinical trials on behalf of pharmaceutical companies. We provide access for thousands of Charleston Lowcountry ...
About. Clinical research trials help us evaluate new drugs and treatments, as well as discover ways to prevent disease. As a participant in a clinical trial, you can have early access to promising therapies before they are widely available. Gibbs Cancer Center is currently participating in more than 150 cancer trials as part of our commitment ...
The Duke Clinical Research Institute, part of the Duke University School of Medicine, is the world's largest academic clinical research organization. We conduct innovative research to deliver on our mission to share knowledge that improves health around the world. DCRI projects are led by renowned experts and physician scientists whose ...
About Clinical Trials; About Pediatric Research; Contact; Through research, new discoveries are made possible is the South Carolina Research Studies Directory designed specifically to help people, like you, locate research studies in which to participate. Tell a friend! Search Studies:
Southern California Clinical and Translational Science Institute (SC CTSI) is a multifaceted resource for clinical and community-partnered translational research. We provide pilot funding, diverse training opportunities, robust clinical research support, digital recruitment methods, community…
High standards of trial conduct & patient care. M3 Wake Research Charleston, as part of the M3 Wake Research network, specializes in conducting clinical trials with a focus on patient safety and scientific integrity. Our collaboration ensures access to a range of therapeutic areas, maintaining the high standards of trial conduct and patient ...
As South Carolina's premier biomedical research institute, we know there's always new terrain to cover. The drive toward innovation that makes an impact propels us forward: leading the state in research funding, we're home to just one of 60 Clinical and Translational Service Award (CTSA) hubs, our researchers published over 3,500 articles ...
Velocity Clinical Research is the world's leading organization of fully integrated research sites. The company partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, and diagnostics that could improve human health and wellbeing. Velocity's unified research site solutions deliver the right patients ...
Clinical Research Institute SC. 666 likes. Somos una empresa dedicada a estudios clínicos, la atención a los pacientes no tiene costo. Nos ca
Southern California Clinical and Translational Science Institute (SC CTSI) is a multifaceted resource for clinical and community-partnered translational research. We provide pilot funding, diverse training opportunities, robust clinical research support, digital recruitment methods, community…
This review introduces a conceptual framework for understanding stakeholder management (ShM) in the clinical and community-based research environment. In recent years, an evolution in practice has occurred in many applicants for public and nongovernmental funding of public health research in hospital settings. Community health research projects are inherently complex, have sought to involve ...
In this article, a working group of expert researchers and clinicians present a set of recommendations to guide the design and conduct of remote medication clinical trials for dementia prevention. Decentralized Clinical Trials for Medications To Reduce the Risk of Dementia: Consensus Report and Guidance | National Institute on Aging
Distinguished Clinical Research Achievement Award — $5,000 is awarded to two studies that show creativity, innovation, or a novel approach that demonstrates an immediate impact on the health and well-being of patients. All Top 10 winners will also be interviewed for the Journal of Clinical and Translational Science.
Phistar Clinical Research Institute, Noida, Uttar Pradesh Application Form, Admissions, Contact, Website, Map, Certification . 2 Courses.
Welcome to the Southern California Clinical and Translational Science Institute (SC CTSI), a multifaceted resource for clinical and community-partnered translational research. Set in the urban heart of Los Angeles where 85% of residents are from minority groups, 90 languages are spoken, and disease burden is high, we support researchers who ...
Hu et al. (2024) 'Functional and Clinical Characterization of Variants of Uncertain Significance Identifies a Hotspot for Inactivating Missense Variants in RAD51C' Cancer Research. DOI: 10.1158/0008-5472.CAN-22-2319
Virtual attendance will be available.An implementation science journal club, where researchers discuss the latest research and their own works-in-progress, will be held on the third Thursday of the month at 1:00 pm in the Institute for Clinical and Translational Science (C44-A General Hospital). The journal club is held as a hybrid meeting.
Southern California Clinical and Translational Science Institute (SC CTSI) is a multifaceted resource for clinical and community-partnered translational research. We provide pilot funding, diverse training opportunities, robust clinical research support, digital recruitment methods, community…
Southern California Clinical and Translational Science Institute (SC CTSI) is a multifaceted resource for clinical and community-partnered translational research. We provide pilot funding, diverse training opportunities, robust clinical research support, digital recruitment methods, community…