Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 February 2024

Persistence in risk and effect of COVID-19 vaccination on long-term health consequences after SARS-CoV-2 infection
- Ivan Chun Hang Lam ORCID: orcid.org/0000-0002-5891-3940 1 na1 ,
- Ran Zhang 2 na1 ,
- Kenneth Keng Cheung Man ORCID: orcid.org/0000-0001-8645-1942 1 , 3 , 4 , 5 ,
- Carlos King Ho Wong ORCID: orcid.org/0000-0002-6895-6071 1 , 2 , 3 , 6 ,
- Celine Sze Ling Chui 3 , 7 , 8 , 9 ,
- Francisco Tsz Tsun Lai ORCID: orcid.org/0000-0002-9121-1959 1 , 2 , 3 , 9 ,
- Xue Li ORCID: orcid.org/0000-0003-4836-7808 1 , 3 , 9 , 10 ,
- Esther Wai Yin Chan ORCID: orcid.org/0000-0002-7602-9470 1 , 3 , 11 , 12 ,
- Chak Sing Lau ORCID: orcid.org/0000-0001-6698-8355 13 ,
- Ian Chi Kei Wong ORCID: orcid.org/0000-0001-8242-0014 1 , 3 , 9 , 14 na2 &
- Eric Yuk Fai Wan ORCID: orcid.org/0000-0002-6275-1147 1 , 2 , 3 , 9 na2
Nature Communications volume 15 , Article number: 1716 ( 2024 ) Cite this article
56k Accesses
13 Citations
718 Altmetric
Metrics details
- Disease prevention
- Epidemiology
- Infectious diseases
The persisting risk of long-term health consequences of SARS-CoV-2 infection and the protection against such risk conferred by COVID-19 vaccination remains unclear. Here we conducted a retrospective territory-wide cohort study on 1,175,277 patients with SARS-CoV-2 infection stratified by their vaccination status and non-infected controls to evaluate the risk of clinical sequelae, cardiovascular and all-cause mortality using a territory-wide public healthcare database with population-based vaccination records in Hong Kong. A progressive reduction in risk of all-cause mortality was observed over one year between patients with SARS-CoV-2 infection and controls. Patients with complete vaccination or have received booster dose incurred a lower risk of health consequences including major cardiovascular diseases, and all-cause mortality than unvaccinated or patients with incomplete vaccination 30-90 days after infection. Completely vaccinated and patients with booster dose of vaccines did not incur significant higher risk of health consequences from 271 and 91 days of infection onwards, respectively, whilst un-vaccinated and incompletely vaccinated patients continued to incur a greater risk of clinical sequelae for up to a year following SARS-CoV-2 infection. This study provided real-world evidence supporting the effectiveness of COVID-19 vaccines in reducing the risk of long-term health consequences of SARS-CoV-2 infection and its persistence following infection.
Similar content being viewed by others
A retrospective cohort study of incidence and risk factors for severe SARS-CoV-2 breakthrough infection among fully vaccinated people

Evaluation of the risk of SARS-CoV-2 infection and hospitalization in vaccinated and previously infected subjects based on real world data

Risk of SARS-CoV-2 infection and hospitalization in individuals with natural, vaccine-induced and hybrid immunity: a retrospective population-based cohort study from Estonia
Introduction.
Since the outbreak of the Coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus in late 2019, substantial research has been undertaken to uncover the health consequences associated with SARS-CoV-2 infection. The current body of evidence has reported that patients with SARS-CoV-2 infection may incur a greater risk of acute and post-acute health consequences involving multiple organ systems and associated mortality following SARS-CoV-2 infection 1 , 2 , 3 .
A universal definition of post-COVID-19 condition remains to be determined owing to the discrepancy in definition published by different healthcare regulatory bodies 4 . Nevertheless, the current literature referred to clinical presentations develop within 30 days of initial infection as acute clinical sequelae, while complications that developed or persisted beyond the acute phase of SARS-CoV-2 infection were referred to as post-acute clinical sequelae 5 . Despite the current evidence suggesting that most patients may recover from SARS-CoV-2 infection within two to four weeks of symptoms appearance, the increased risk of incident cardiovascular, neurological, psychiatric diseases, diabetes and all-cause mortality was shown to persist for up to two years 6 , 7 , 8 , 9 , 10 . Patients with a severe SARS-CoV-2 infection or critically ill patients are at particular risk of developing long-term adverse outcome beyond their acute infection. Nevertheless, evidence emerged from the earlier stage of the pandemic were limited by the selection of under-represented samples as the study population 11 . Moreover, findings reported from the current body of literature were based largely on the assumption of a constant risk increase of clinical sequelae over a prolonged duration which may not be able to account for the change in risk over time, thus hindering the representativeness on the burden of the long-term health consequences of SARS-CoV-2 infection. Such speculation was evident from the gradual improvement in pulmonary function in most patients who recovered from severe SARS-CoV-2 infection over a three-monthly observation period for 12 months 12 . Meanwhile, a recent study in Israel have reported a considerably reduced risk of PASC towards the later period of SARS-CoV-2 infection over a course of one year amongst patients with mild infection 13 .
Shortly after the outbreak of the pandemic, the global initiative to develop vaccines against SARS-CoV-2 infection followed by the international vaccination campaign has been shown to successfully reduce the risk of primary infection, disease severity and hospitalization associated with SARS-CoV-2 infection 14 . A two dose vaccination regimen was initially recommended for the vast majority of brand of COVID-19 vaccines including the BioNtech and CoronaVac offered in Hong Kong based on the efficacy profile in preventing primary infection established from earlier clinical trials 15 , 16 . Nevertheless, a third and even fourth booster dose of the vaccine was later introduced in certain countries to restore immunity within the population in the face of the Omicron variant of SARS-CoV-2 17 , 18 . Despite the cumulative evidence of the covid-19 vaccines’ ability to reduce disease burden during acute infection, its effect in preventing the adverse outcome in the post-acute phase of SARS-CoV-2 infection remained largely unknown owing to the inconsistent findings from the existing studies 19 , 20 . While a protective effect of COVID-19 vaccination on incident health outcomes has been reported in several population and community-based studies, the extent of risk reduction, especially from the booster dose of vaccines remains to be evaluated 21 , 22 .
This population-based study aims to evaluate the progressive risk of health consequences associated with SARS-CoV-2 infection over 1 year and compare the risk and persistence of such risk differences between patients of different COVID-19 vaccination statuses.
A total of 1,175,277 patients with SARS-CoV-2 infection were identified in this study, of those, 124,443, 101,379, 457,896, and 491,559 patients were unvaccinated, had one, two and three or more doses of COVID-19 vaccination record prior to infection, respectively (Fig. 1 ). The median time between the latest dose of vaccination and SARS-CoV-2 infection for patients who have received one, two and three or more doses of COVID-19 vaccination was 18 (interquartile range 12–42), 175 (119–208) and 101 (37–170) days, respectively. The median follow-up period for controls, patients with SARS-CoV-2 who were unvaccinated, have received one, two and three or more doses of COVID-19 vaccination were 318 (173–329), 320 (253–331), 324 (312–329), 325 (312–330), and 171 (135–316) days, respectively. The baseline characteristics before and after weighting were summarized in Tables 1 and 2 , respectively. The SMDs of all baseline characteristics after weighting were less than 0.1, indicating a good balance between groups of patients with different vaccination statuses. The number of patients at risk at each observation period between days 0–30, 31–90, 91–180, 181–270, 271–365 were 2,815,023, 2,801,396, 2,764,243, 2,119,507, and 1,876,858, respectively.
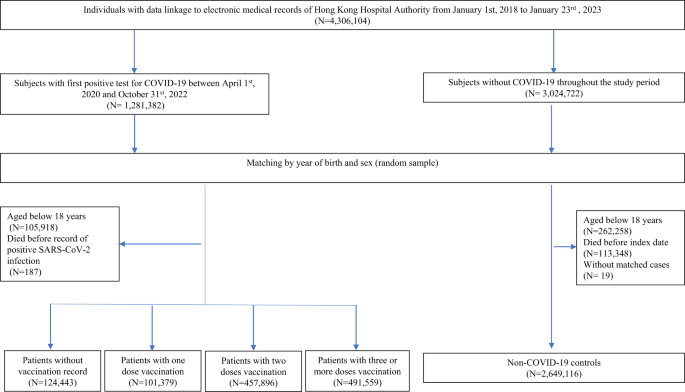
Flowchart for identifying study population.
Overall, a graded reduction in the risk of clinical sequelae among infected patients was observed over time and with the increased number of doses of COVID-19 vaccines received prior to infection. Compared to non-infected controls, patients with SARS-CoV-2 infection were observed to incur a greater risk of clinical sequelae including major cardiovascular diseases [HR dose=0: 4.64 (4.00, 5.38); dose=1: 3.13 (2.60, 3.76); dose=2: 2.53 (2.21, 2.89); dose ≥3: 1.99 (1.72, 2.29)], and all-cause mortality [HR dose=0: 18.89 (18.07, 19.74); dose=1: 8.96 (8.46, 9.48); dose=2: 3.95 (3.71, 4.20); dose ≥3: 1.74 (1.50, 2.02)] across patients with different COVID-19 vaccination status during the acute phase of infection. A graded decrease in risk was observed in patients with a greater number of doses of COVID-19 vaccination with unvaccinated and incompletely vaccinated patients incurring a greater risk of most clinical sequelae than those completely vaccinated and those who received booster doses of vaccines. Most notably, there was an approximately five-fold reduction in risk of all-cause mortality between unvaccinated patients (18.89; 18.07,19.74) and patients with complete vaccination (3.95; 3.71, 4.20) during the acute phase of infection. Such risk was further reduced amongst patients who received booster dose of vaccines (1.74; 1.50, 2.02).
The risk of all-cause mortality reduced progressively over one year between patients with SARS-CoV-2 infection and non-COVID-19 controls [0–30 d: 18.89 (18.07, 19.74); 31–90 d: 3.79 (3.56, 4.03); 91–180 d: 2.11 (1.97, 2.26); 181-270d: 1.97 (1.83, 2.13); 271–365 d: 2.12 (1.92, 2.33)]. A sharp decline in the risks of the outcomes were observed during the post-acute phase between 31 and 90 days of infection compared to the acute phase especially in patients who received complete and booster dose of vaccination. Nevertheless, the risk of several clinical sequelae including heart failure (dose=2: 1.43; 1.13, 1.81; dose ≥3: 1.35; 1.05, 1.75) and all-cause mortality (dose=2: 1.11; 1.02, 1.21) remained significantly greater in patients with SARS-CoV-2 infection. The risk of clinical sequelae further reduced in the subsequent observation period with no significant greater risk of clinical sequelae observed among completely vaccinated and patients with booster dose of vaccines from 271 and 91 days onwards, respectively. Meanwhile, the increased risk of certain clinical sequelae including all-cause mortality (2.12; 1.92, 2.33) continued to persist for up to a year amongst unvaccinated patients. (Tables 3 – 7 and Fig. 2 ) The cumulative incidence plots for individual observation windows were shown in Supplementary Fig. 1 . Sensitivity analyses reported largely consistent findings for the aforementioned outcomes (Supplementary Tables 2 – 11 ). A moderate increase in risk of lung cancer and lymphoma was observed during the first 30 days of infection. (Supplementary Table 12 ).
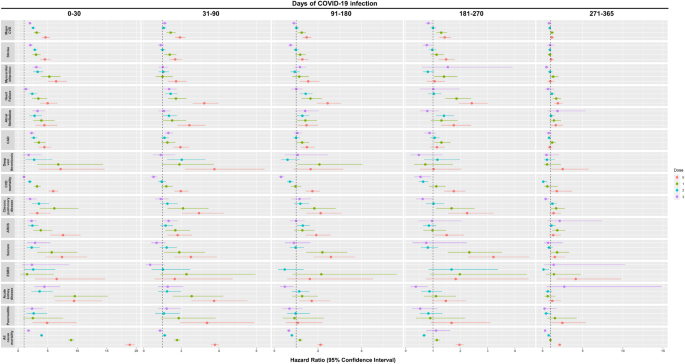
Note: Major CVD major cardiovascular disease, Composite outcome of stroke, heart failure and coronary artery disease, ARDS acute respiratory distress syndrome, CAD coronary artery disease, ESRD end-stage renal disease. Hazard ratio (HR) and 95% confidence interval (95% CI) were estimated by Cox regression, HR > 1 (or <1) indicates patients with COVID-19 had higher (lower) risk of sequelae compared to the non-COVID-19 control cohort. Dose 0, 1, 2, 3 refers to study population who have received 0 dose, 1 dose, 2 doses, and ≥3 doses of BioNtech or CoronaVac COVID-19 vaccines, respectively.
Patients aged above 65 or with a CCI of four or above are at a greater risk of clinical sequelae during the acute and post-acute phase of SARS-CoV-2 infection especially amongst unvaccinated or incompletely vaccinated patients. Male and female incurred broadly comparable risk of acute and post-acute sequelae with respect to their vaccination status. (Supplementary Tables 13 – 18 ).
This study examined the risk of long-term health consequences of SARS-CoV-2 infection involving multiple organ systems between patients with a history of SARS-CoV-2 infection and non-infected controls over the course of 365 days. The findings of this study showed a reduction in the risk of the majority of clinical sequelae over the course of the observation window suggesting the gradual subside of the risk of long-term health consequences over a year. COVID-19 vaccination, especially the uptake of the booster dose, was found to be effective in reducing the risk of health consequences. Patients who received three or more doses of vaccines did not incur any significant risk increased in clinical sequelae from 91 days onwards from their initial infection. On the other hand, unvaccinated patients were at a greater risk of several clinical sequelae including all-cause mortality up to one year following infection. Such findings provided evidence to support the potential protection from vaccination in reducing the risk of long-term health consequences following SARS-CoV-2 infection.
Previous studies that characterized and evaluated the risk of post-acute sequelae have reported a considerable increase in risk of clinical sequelae involving multiple organ systems 1 , 2 , 7 , 8 , 10 . In particular, patients with a severe SARS-CoV-2 infection during the acute phase were reported to incur further risk of post-acute clinical sequelae following infection 23 . Nevertheless, evidence that emerged in the early stage of the pandemic has been generated based on data with limited representation of the general population such as discharged hospitalized and older patients with SARS-CoV-2 infection. Since these factors were indicative of patients with more severe disease outcomes compared to the general population, the findings from these studies have shown great discrepancies in the prevalence and risk of clinical sequelae following infection 11 , 24 . More recent evidence from several large-scale, nationwide population-based studies have indicated a gradual improvements in their recovery status characterized by the reduction in prevalence of self-reported symptoms and proportion of infected individuals reporting non-recovery from health outcomes associated with SARS-CoV-2 infection 25 , 26 . A reduction in risk of post-infection clinical sequelae was also reported 6 months following their initial infection amongst individuals with a mild SARS-CoV-2 infection 13 . With consideration of the comparable disease severity induced by the dominant XBB variant to the other sublineages of the omicron strain of SARS-CoV-2, the current body of evidence may indicate the gradual subside and lesser clinical burden on the long-term health consequences caused by such milder strain of SARS-CoV-2 virus than that reported in the earlier stage of the pandemic 27 . Nonetheless, high-risk patients including older people, lack of vaccination, immunosuppression, and individuals with certain underlying comorbidities may still be more vulnerable to poor clinical prognosis from the adverse clinical outcomes following SARS-CoV-2 infection. This emphasizes the need for closer monitoring for these patient groups and provide timely treatment if necessary.
As the pandemic evolved, the global COVID-19 vaccination campaigns have shown to be effective in preventing serious illness and death associated with SARS-CoV-2 infection. Recent research on the booster dose of COVID-19 vaccination has reported substantial extra protection against initial, severe SARS-CoV-2 infection and the rate of mortality by over 80% with a greater risk reduction estimated amongst high-risk population with multi-morbidity, in addition to that conferred by the first and second doses 28 , 29 , 30 , 31 . The bivalent omicron-containing booster vaccine was also shown to confer broader immunity against the Omicron variant of SARS-CoV-2 and reduce hospitalizations or deaths due to SARS-CoV-2 infection 32 , 33 . Previous studies investigating the impact of COVID-19 vaccines on the risk of developing clinical sequelae associated with SARS-CoV-2 infection have reported inconsistent findings due to the difference in the methodological approach employed to evaluate two key aspects: the effect of vaccination on the risk of incident clinical sequelae following SARS-CoV-2 infection and the prevalence of post-infection sequelae outcomes among COVID-19 survivors 19 , 20 . Further to the current evidence supporting the protection against both acute and post-acute health consequences of SARS-CoV-2 infection conferred by COVID-19 vaccination, the findings of this study demonstrated a graded reduction in the incidence and the persistence in risk according to the number of vaccines doses received prior to infection. The protective effect was more pronounced among the older people and patients with a greater degree of morbidity. Nevertheless, further studies are warranted to evaluate the effect of COVID-19 vaccination in reducing the prevalence of clinical sequelae and understand the potential protective effect of COVID-19 vaccination in patients who have developed onset of clinical sequelae associated with SARS-CoV-2 infection.
Despite the recapitulation of evidence demonstrating the association of COVID-19 vaccination with a reduced risk of clinical sequelae and adverse clinical outcomes during the acute phase of SARS-CoV-2 infection, its protective effect against clinical sequelae in the post-acute phase of infection remained largely unknown. One putative mechanism for the protection effects against the long-term health consequences from vaccination observed could be attributed to the protection against severe SARS-CoV-2 infection during the acute infection may subsequently reduce the risk of long-term health outcomes. The most common COVID-19 vaccines development approach was based on the spike protein of the virus as an antigen accessible by antibodies and immunological cells in the body. The BioNtech COVID-19 vaccines contains mRNA molecules carrying the information for the SARS-CoV-2 spike protein whilst the CoronaVac consisted of whole attenuated SARS-CoV-2 virus capable of provoking an immune response upon administration. The spike proteins attracts antibodies and provoke a high response of sub-types CD8+ killer and CD4 helper T cells, signaling the production of cytokines and proliferations of T memory cells to mediate a lasting immunity, preventing severe health consequences from SARS-CoV-2 infection and the associated irreversible damage to vital organ systems in our body, constituting to a reduced risk of subsequent clinical sequelae beyond the acute phase of SARS-CoV-2 infection 34 .
The findings of this study demonstrated a gradual reduction in the risk of long-term health consequences associated with SARS-CoV-2 over one year, indicating a lesser disease burden compared to that reported in earlier studies as well as the effect of COVID-19 vaccination in reducing the risk of clinical sequelae beyond the acute phase of SARS-CoV-2 infection. The comprehensive records of vaccination records provided by the Department of Health ensures the accuracy of information on the vaccination status of individuals reported in this study. As the pandemic progresses, our findings provided real-world evidence supporting the effectiveness of the COVID-19 vaccines in the prevention of long-term health consequences following SARS-CoV-2 infection. Nevertheless, our study is subject to several limitations. First, detection bias might be inherent in this study due to the potential under-reporting of existing underlying conditions prior to a diagnosis of SARS-CoV-2 infection. In addition, the increased healthcare contacts from receiving further examination amongst patients diagnosed with SARS-CoV-2 infection could result in the increased diagnosis of condition which could have persisted prior to infection. For instance, patients presenting with sequelae may have developed certain diseases prior to their diagnosis of SARS-CoV-2 infection; yet they did not receive a diagnosis for those conditions until a confirmed diagnosis of SARS-CoV-2 infection, resulting in the over-attribution of disease diagnosis as post-infection sequelae. Nevertheless, the history of chronic diseases in the HKHA has been recorded with high completeness as demonstrated in previous study, thus ensuring the accuracy and reliability of data to distinguish existing comorbidities and sequelae of SARS-CoV-2 infection 35 . Given the sufficiently long observation period, any existing comorbidities of subjects that were not captured is considered unlikely. Such error would also have a minimal effect on sequelae observed during the post-acute phase of infection. Furthermore, sequelae reported in this study including stroke, MI and seizure were largely of great disease severity which would typically result in distinct symptoms upon the onset of disease. Thus, the incidence of such sequelae would not be affected by the increased surveillance on patients following SARS-CoV-2 infection. Second, potential selection bias may also arise from the increased SARS-CoV-2 testing amongst individuals with prevalent comorbidities. Third, the emergence of novel variant of SARS-CoV-2 is strongly correlated with better vaccination coverage and booster dose of vaccination. Given the lower severity and risk of health consequences associated with Delta and Omicron variant emerged later in the pandemic, this could lead to potential confounding bias in our findings. Nevertheless, sensitivity analysis which adjusted for the likely variant of SARS-CoV-2 amongst the infected patients have reported a largely consistent result as the main analysis suggesting that such potential confounding bias should not impact the study’s overall conclusions. Fourth, the estimation of period-specific HR is subjected to possible built-in selection bias from the censoring of patients upon the incident of clinical sequelae and the systematic difference in distribution of unknown ubiquitous factors between survivors from separate cohorts exist generally especially toward the later stage of follow-up. This could contribute to the reduction in the magnitude of HR estimated in the later observation windows. Further study is warranted to evaluate the effect of the built-in selection bias described 36 , 37 . Lastly, residual confounding bias can remain even after weighing subjects according to their propensity scores. Several important unmeasured confounders, namely obesity, smoking, socioeconomic status, educational level and strains of SARS-CoV-2 virus found in individual patients, could not be accounted for in this study owing to data availability, which may have introduced bias to our results.
This study examined the progressive risk of acute and post-acute sequelae following SARS-CoV-2 infection at 3 monthly intervals up to a year amongst patients with different vaccination status. The risk of clinical sequelae was observed to reduce gradually over the observation period. Complete vaccination and the uptake of booster dose of COVID-19 vaccines were found to further reduce the risk and persistence in risk of long-term health consequences of SARS-CoV-2 infection. The findings of this study indicated a lesser disease burden caused by health consequences of SARS-CoV-2 infection compared to that reported in earlier study and provided real-world evidence supporting the effectiveness of COVID-19 vaccines in reducing the risk of long-term health consequences following infection.
Data source
In this retrospective cohort study, routine electronic medical records were retrieved from the Hong Kong Hospital Authority (HKHA). The Hospital Authority is a statutory body that manages all public hospitals and their ambulatory clinics in Hong Kong. The service is available to all HK residents (> 7.2 million) covering ~80% of all routine hospital admissions 38 . Electronic medical records from the HKHA database consisted of disease diagnoses recorded in planned or unplanned doctor consultations from in- and outpatient hospitals and emergency visits, thus allowing timely capture of all medical records of all users of the public health services in HK. Records were obtained from the Hong Kong Deaths Registry to identify mortality in this study. Information on vaccination status was provided by the Department of Health, The Government of Hong Kong Special Administrative Region whilst records of confirmed cases of SARS-CoV-2 infection were obtained from the Centre for Health Protection of the Government, the Hong Kong Special Administrative Region and HKHA. Anonymized unique patient identifiers were used to integrate these databases. These population-based databases have been used in previous studies on the long-term sequelae of COVID-19 infection, COVID-19 vaccines safety surveillance and effectiveness 3 , 6 , 38 , 39 , 40 , 41 , 42 .
Study design and population
Individuals with data linkage to electronic medical records of Hong Kong Hospital Authority from January 1, 2018 to January 23, 2023 were eligible for this study. A cohort study was conducted to evaluate the risk of health consequences between patients with and without SARS-CoV-2 infection aged 18 years or above. Patients with an incident SARS-CoV-2 infection (confirmed by rapid antigen test [RAT] or polymerase chain reaction [PCR] test in throat swab, nasopharyngeal aspirate, or deep throat sputum specimens) between April 1, 2020 and October 31, 2022 were matched to non-infected controls without a positive SARS-CoV-2 test record throughout the study period with the exact birth-year and sex. All individuals without a record of positive test record of the same birth-year and sex were selected as matched controls. Patients with SARS-CoV-2 infection were further stratified into (1) unvaccinated (0 dose), (2) incompletely vaccinated (1 dose), (3) completely (2 doses), and (4) vaccinated with booster doses ( ≥ 3 doses) according to the number of BioNtech or CoronaVac vaccines received prior to first SARS-CoV-2 infection. The index date of patients with SARS-CoV-2 infection was defined as the date of first diagnosis date of SARS-CoV-2 infection. The identical index date was assigned to randomly selected corresponding matched controls as the pseudo-index date.
All subjects were followed up from the index date until the date of death, the occurrence of outcome, SARS-CoV-2 re-infection or the end of the separate observation periods at 30, 90, 180, 270, and 365 days after the index date or the end of the study period January 31, 2023, whichever occurred earlier.
Anonymized longitudinal clinical healthcare data since 2016 and the earliest date of data availability were obtained for all subjects from HKHA. Relevant data included baseline demographic (sex, age and Charlson Comorbidity Index); pre-existing morbidities captured by clinical diagnosis codes (cardiovascular, cerebrovascular, respiratory, chronic kidney, liver diseases, rheumatoid arthritis and malignancy; Supplementary Table 1 ), history of long-term medication (renin–angiotensin-system agents, beta-blockers, calcium channel blockers, diuretics, nitrates, lipid-lowering agents, insulins, antidiabetic drugs, oral anticoagulants, antiplatelets and immunosuppressants) and COVID-19 vaccination status before index date.
This study was reported according to the Reporting of studies Conducted using Observational Routinely-collected Data (RECORD), extended from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
Outcomes of clinical diagnosis
The outcomes of this study were selected based on previous evidence on the risk of clinical sequelae associated with SARS-CoV-2 infection which includes incidences of major cardiovascular diseases (a composite outcome of stroke, heart failure and coronary heart disease), stroke, myocardial infarction (MI), heart failure, atrial fibrillation, coronary artery disease, deep vein thrombosis (DVT), chronic pulmonary disease, acute respiratory distress syndrome, seizure, end-stage renal disease, acute kidney injury, pancreatitis, cardiovascular and all-cause mortality 1 , 8 , 9 , 10 , 43 . Outcomes were identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM; Supplementary Table 1 ).
Statistical analyses
Inverse Probability Treatment Weighting (IPTW) 44 based on age, sex, Charlson Comorbidity index (CCI), history of separate class of medication (renin–angiotensin system agents, beta-blockers, calcium channel blockers, diuretics, nitrates, lipid-lowering agents, insulins, antidiabetic drugs, oral anticoagulants, antiplatelets and immunosuppressants), the number of hospital admission and doctor consultation within one year of index date was applied to account for potential confounding factors. Standardized mean difference (SMD) between cases and controls was estimated, SMD ≤ 0.1 was regarded as sufficient balance between case and control groups 45 . Subjects with a history of outcome of interest were excluded from the analysis of the specific conditions whilst continued to be considered at risk for other disease outcomes. The incidence rate (per 1000 person-years), hazard ratio (HR) and 95% confidence interval (CI) of each outcome were estimated between COVID and non-COVID-19 cohorts separately for each of the observation period using Cox proportional hazard regressions. Sensitivity analysis was performed by only including individuals with a positive PCR SARS-CoV-2 screening test results, cases of SARS-CoV-2 infection from the Omicron wave in Hong Kong 46 , unvaccinated patients with COVID-19 and matched control with the same vaccination status, adjusting for the likely variant of SARS-CoV-2 responsible for the infection, excluding patients who received their last dose of vaccine more than 6 months before SARS-CoV-2 infection owing to the waning of immunity following vaccination 47 , 48 , and controlling for the false discovery rate at 0.05 through Benjamin-Hochberg procedure 49 . Lung cancer, brain cancer, and lymphoma which were considered to have a prolonged latent period for their development were included as negative control outcomes to detect possible testing bias. Subgroup analyses were predefined taking account of the risk factors of post-COVID-19 condition 50 . Patients were stratified by (1) age (≤65, >65), (2) sex, (3) Charlson Comorbidity index (CCI; <4, ≥4).
All statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). All significance tests were two‐tailed. A P value less than 0.05 or 95% CI excluding 1.0 were taken to indicate statistical significance. At least two investigators (ICHL, RZ, and EYFW) conducted each of the statistical analyses independently for quality assurance.
Data access
EYFW and ICKW had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical approval
Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (UW20-556 and UW21-149) and Department of Health, HK (L/M21/2021 and L/M175/2022) with an exemption for informed consent from participants as patients’ confidentiality was maintained in this retrospective cohort study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data contains confidential information and hence cannot be shared with the public due to third-party use restrictions. Local academic institutions, government departments, or non-governmental organizations may apply for the access to data through the Hospital Authority’s data-sharing portal ( https://www3.ha.org.hk/data ).
Code availability
The code used in this study is available on Zenodo ( https://doi.org/10.5281/zenodo.10132693 ).
Daugherty, S. E. et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 373 , n1098 (2021).
Article PubMed Google Scholar
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594 , 259–264 (2021).
Article ADS PubMed CAS Google Scholar
Lam, I. C. H. et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. eClinicalMedicine 60 , 102000 (2023).
Article PubMed PubMed Central Google Scholar
Chaichana, U. et al. Definition of post-COVID-19 condition among published research studies. JAMA Netw. Open 6 , e235856–e235856 (2023).
Thaweethai, T. et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 329 , 1934–1946 (2023).
Article PubMed PubMed Central CAS Google Scholar
Wan, E. Y. F. et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: a prospective cohort in UK Biobank. Cardiovasc. Res. 119 , 1718–1727 (2023).
Taquet, M. et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 9 , 815–827 (2022).
Xie, Y. & Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 10 , 311–321 (2022).
Xie, J. et al. Clinical and genetic risk factors for acute incident venous thromboembolism in ambulatory patients with COVID-19. JAMA Intern. Med. 182 , 1063–1070 (2022).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 28 , 2406–2415 (2022).
Wu, Q., Ailshire, J. A. & Crimmins, E. M. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci. Rep. 12 , 11647 (2022).
Article ADS PubMed PubMed Central CAS Google Scholar
Wu, X. et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir. Med. 9 , 747–754 (2021).
Mizrahi, B. et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 380 , e072529 (2023).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New Engl. J. Med. 384 , 1412–1423 (2021).
Article PubMed CAS Google Scholar
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl. J. Med. 383 , 2603–2615 (2020).
Tanriover, M. D. et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 398 , 213–222 (2021).
Menni, C. et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 22 , 1002–1010 (2022).
Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. New Engl. J. Med. 386 , 1532–1546 (2022).
Byambasuren, O., Stehlik, P., Clark, J., Alcorn, K. & Glasziou, P. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2 , e000385 (2023).
Notarte, K. I. et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine 53 , 101624 (2022).
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28 , 1461–1467 (2022).
Ayoubkhani, D. et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infect. Dis. 9 , ofac464 (2022).
Xie, Y., Bowe, B. & Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 12 , 6571 (2021).
Ballering, A. V., van Zon, S. K. R., olde Hartman, T. C. & Rosmalen, J. G. M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 400 , 452–461 (2022).
Ballouz, T. et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ 381 , e074425 (2023).
Hastie, C. E. et al. Natural history of long-COVID in a nationwide, population cohort study. Nat. Commun. 14 , 3504 (2023).
Mahase, E. Covid-19: what do we know about XBB.1.5 and should we be worried? BMJ 380 , p153 (2023).
Article Google Scholar
Arbel, R. et al. BNT162b2 vaccine booster and mortality due to Covid-19. New Engl. J. Med 385 , 2413–2420 (2021).
Bar-On, Y. M. et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. New Engl. J. Med 385 , 1393–1400 (2021).
Magen, O. et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. New Engl. J. Med 386 , 1603–1614 (2022).
Barda, N. et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 398 , 2093–2100 (2021).
Arbel, R. et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect. Dis. 23 , 914–921 (2023).
Moreira, E. D. et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. New Engl. J. Med. 386 , 1910–1921 (2022).
Mascellino, M. T., Di Timoteo, F., De Angelis, M. & Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect. Drug Resist. 14 , 3459–3476 (2021).
Wong, M. C. S. et al. Health services research in the public healthcare system in Hong Kong: an analysis of over 1 million antihypertensive prescriptions between 2004–2007 as an example of the potential and pitfalls of using routinely collected electronic patient data. BMC Health Serv. Res. 8 , 138 (2008).
Hernán, M. A. The hazards of hazard ratios. Epidemiology 21 , 13–15 (2010).
Bartlett, J. W. et al. The hazards of period specific and weighted hazard ratios. Stat. Biopharm. Res. 12 , 518–519 (2020).
Lai, F. T. T. et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Ann. Intern. Med. 175 , 362–370 (2022).
Wan, E. Y. F. et al. Safety of an inactivated, whole-virion COVID-19 vaccine (CoronaVac) in people aged 60 years or older in Hong Kong: a modified self-controlled case series. Lancet Healthy Longev. 3 , e491–e500 (2022).
Yan, V. K. C. et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study. Emerg. Microbes Infect. 11 , 2304–2314 (2022).
Yan, X. et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J. Infect. 83 , 381–412 (2021).
Wan, E. Y. F. et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect. Dis. 22 , 64–72 (2022).
Cohen, K. et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 376 , e068414 (2022).
Ruth, C. et al. Long-Term Outcomes Of Manitoba’s Insight Mentoring Program: A Comparative Statistical Analysis (Manitoba Centre for Health Policy, Winnipeg, MB, 2015).
Austin, P. C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 38 , 1228–1234 (2009).
Article MathSciNet Google Scholar
Xie, R. et al. Resurgence of Omicron BA.2 in SARS-CoV-2 infection-naive Hong Kong. Nat. Commun. 14 , 2422 (2023).
Feikin, D. R. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 399 , 924–944 (2022).
Ponticelli, D. et al. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel Med. 28 , taab173 (2021).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57 , 289–300 (1995).
MathSciNet Google Scholar
Subramanian, A. et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 28 , 1706–1714 (2022).
Download references
Acknowledgements
The authors thank the Hospital Authority for the generous provision of data for this study. This work was supported by HMRF Research on COVID-19, The Hong Kong Special Administrative Region (HKSAR) Government (Principal Investigator: EWYC; Ref No. COVID1903011); Collaborative Research Fund, University Grants Committee, the HKSAR Government (Principal Investigator: ICKW; Ref. No. C7154-20GF); and Research Grant from the Health Bureau, the HKSAR Government (Principal Investigator: I.C.K.W.; Ref. No. COVID19F01). I.C.K.W. and F.T.T.L. are partially supported by the Laboratory of Data Discovery for Health (D 2 4H) funded by the AIR@InnoHK administered by the Innovation and Technology Commission. The funders did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
These authors contributed equally: Ivan Chun Hang Lam, Ran Zhang.
These authors jointly supervised this work: Ian Chi Kei Wong, Eric Yuk Fai Wan.
Authors and Affiliations
Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Ivan Chun Hang Lam, Kenneth Keng Cheung Man, Carlos King Ho Wong, Francisco Tsz Tsun Lai, Xue Li, Esther Wai Yin Chan, Ian Chi Kei Wong & Eric Yuk Fai Wan
Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Ran Zhang, Carlos King Ho Wong, Francisco Tsz Tsun Lai & Eric Yuk Fai Wan
Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, Sha Tin, Hong Kong SAR, China
Kenneth Keng Cheung Man, Carlos King Ho Wong, Celine Sze Ling Chui, Francisco Tsz Tsun Lai, Xue Li, Esther Wai Yin Chan, Ian Chi Kei Wong & Eric Yuk Fai Wan
Research Department of Practice and Policy, School of Pharmacy, University College London, London, UK
Kenneth Keng Cheung Man
Centre for Medicines Optimisation Research and Education, University College London Hospitals NHS Foundation Trust, London, UK
Department of Infectious Disease Epidemiology & Dynamics, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK
Carlos King Ho Wong
School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong SAR, Hong Kong, China
Celine Sze Ling Chui
School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Advanced Data Analytics for Medical Science (ADAMS) Limited, Hong Kong, China
Celine Sze Ling Chui, Francisco Tsz Tsun Lai, Xue Li, Ian Chi Kei Wong & Eric Yuk Fai Wan
Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Department of Medicine, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Esther Wai Yin Chan
The University of Hong Kong Shenzhen Institute of Research and Innovation, Hong Kong SAR, China
Division of Rheumatology and Clinical Immunology, Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Chak Sing Lau
Aston Pharmacy School, Aston University, Birmingham, UK
Ian Chi Kei Wong
You can also search for this author in PubMed Google Scholar
Contributions
I.C.H.L., E.Y.F.W., and I.C.K.W. had the original idea for the study, contributed to the development of the study, extracted data from the source database, constructed the study design and the statistical model, reviewed the literature, and act as guarantors for the study. I.C.H.L., R.Z., and E.Y.F.W. accessed and verified the data, and performed statistical analysis. I.C.H.L., R.Z., E.Y.F.W., and I.C.K.W. wrote the first draft of the manuscript. I.C.K.W. is the principal investigator and provided oversight for all aspects of this project. K.K.C.M., C.K.H.W., C.S.L.C., F.T.T.L., X.L., E.W.Y.C., C.S.L., E.Y.F.W., and I.C.K.W. provided critical input to the analyses, study design, and discussion. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript to be submitted.
Corresponding authors
Correspondence to Ian Chi Kei Wong or Eric Yuk Fai Wan .
Ethics declarations
Competing interests.
K.K.C.M. reported grants from the Hong Kong Research Grant Council, the CW Maplethorpe Fellowship, UK National Institute for Health and Care Research, European Commission Framework Horizon 2020, Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region, and personal fees from IQVIA Ltd outside the submitted work. CKHW. reports the receipt of General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation; AstraZeneca and Boehringer Ingelheim, all outside the submitted work. C.S.L.C. has received grants from the Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. F.T.T.L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. X.L. has received research grants from Hong Kong Health and Medical Research Fund (HMRF, HMRF Fellowship Scheme, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fees from Merck Sharp & Dohme and Pfizer, unrelated to this work. E.W.C. reports grants from Research Grants Council (RGC, Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region; honorarium from Hospital Authority; outside the submitted work. ICKW reports grants from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK and Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund in Hong Kong, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, consulting fees from IQVIA and World Health Organization, payment for expert testimony for Appeal Court of Hong Kong and is a non-executive director of Jacobson Medical in Hong Kong and Therakind in England, outside of the submitted work; no other relationships or activities that could appear to have influenced the submitted work. EYFW has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region, and the Hong Kong Research Grants Council, outside the submitted work. The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lam, I.C.H., Zhang, R., Man, K.K.C. et al. Persistence in risk and effect of COVID-19 vaccination on long-term health consequences after SARS-CoV-2 infection. Nat Commun 15 , 1716 (2024). https://doi.org/10.1038/s41467-024-45953-1
Download citation
Received : 24 August 2023
Accepted : 08 February 2024
Published : 26 February 2024
DOI : https://doi.org/10.1038/s41467-024-45953-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 19 May 2023
The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis
- Marzieh Soheili 1 ,
- Sorour Khateri 2 ,
- Farhad Moradpour 3 ,
- Pardis Mohammadzedeh 4 ,
- Mostafa Zareie 4 ,
- Seyede Maryam Mahdavi Mortazavi 5 ,
- Sima Manifar 6 ,
- Hamed Gilzad Kohan 7 &
- Yousef Moradi 3
Annals of Clinical Microbiology and Antimicrobials volume 22 , Article number: 42 ( 2023 ) Cite this article
18k Accesses
188 Altmetric
Metrics details
This meta-analysis evaluated the Efficacy and Effectiveness of several COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson, to better estimate their immunogenicity, benefits, or side effects.
Studies reporting the Efficacy and Effectiveness of COVID-19 vaccines from November 2020 to April 2022 were included. The pooled Effectiveness/Efficacy with a 95% confidence interval (95% CI) with Metaprop order was calculated. The results were presented in forest plots. Predefined subgroup analyses and sensitivity analyses were also performed.
A total of twenty articles were included in this meta-analysis. After the first dose of the vaccine, the total effectiveness of all COVID-19 vaccines in our study was 71% (95% CI 0.65, 0.78). The total effectiveness of vaccines after the second dose was 91% (95% CI 0.88, 0.94)). The total efficacy of vaccines after the first and second doses was 81% (95% CI 0.70, 0.91) and 71% (95% CI 0.62, 0.79), respectively. The effectiveness of the Moderna vaccine after the first and second dose was the highest among other studied vaccines ((74% (95% CI, 0.65, 0.83) and 93% (95% CI, 0.89, 0.97), respectively). The highest first dose overall effectiveness of the studied vaccines was against the Gamma variant (74% (95% CI, 0.73, 0.75)), and the highest effectiveness after the second dose was observed against the Beta variant (96% (95% CI, 0.96, 0.96)). The Efficacy for AstraZeneca and Pfizer vaccines after the first dose was 78% (95% CI, 0.62, 0.95) and 84% (95% CI, 0.77, 0.92), respectively. The second dose Efficacy for AstraZeneca, Pfizer, and Bharat was 67% (95% CI, 0.54, 0.80), 93% (95% CI, 0.85, 1.00), and 71% (95% CI, 0.61, 0.82), respectively. The overall efficacy of first and second dose vaccination against the Alfa variant was 84% (95% CI, 0.84, 0.84) and 77% (95% CI, 0.57, 0.97), respectively, the highest among other variants.
mRNA-based vaccines against COVID-19 showed the highest total efficacy and effectiveness than other vaccines. In general, administering the second dose produced a more reliable response and higher effectiveness than a single dose.
Introduction
The coronavirus disease 2019 (COVID-19) is an acute respiratory infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This β-coronavirus is an enveloped, non-segmented positive-sense RNA virus, which primarily spreads through the respiratory tract [ 1 , 2 , 3 ]. COVID-19 infection is often associated with systemic inflammation and inflammatory biomarkers such as IL-6, IL-10, and TNF-α) increase in the patients [ 4 , 5 , 6 ]. Cough, fever, and shortness of breath are the dominant symptoms of COVID-19 infection. Additionally, fatigue, increased sputum production, sore throat, headache, and gastrointestinal symptoms might be observed [ 6 , 7 , 8 ]. Elderly patients with underlying disorders such as hypertension, chronic obstructive pulmonary disease, diabetes, and cardiovascular complications are more prone to develop acute respiratory distress syndrome. Other severe symptoms include septic shock, metabolic acidosis, and coagulation dysfunction, which might lead to death [ 9 , 10 ]. Various medications have already been tested for treating COVID-19 patients. However, the evidence to support the beneficial effects of these drugs is often controversial [ 11 , 12 , 13 ]. Molnupiravir is the first oral antiviral drug that has recently shown a significant benefit in reducing hospitalization or death in COVID-19 patients [ 14 ].
According to the World Health Organization (WHO) report, from the emergence of COVID-19 in December 2019 to November 2021, more than 250,000,000 confirmed cases of COVID-19 have been reported, and more than five million deaths have been attributed to the disease globally [ 15 ]. Since the COVID-19 pandemic, several studies have started to develop safe and efficacious vaccines. Numerous clinical trials have been conducted to evaluate the efficacy and safety of experimental vaccines [ 16 , 17 , 18 ]. WHO reported as of November 8, 2021, more than seven billion vaccine doses have been administered worldwide [ 15 ]. Additionally, as per the WHO report, until November 9, 2021, 130 vaccine candidates were under clinical development, and 156 candidates were in the pre-clinical development phase. Different types of COVID-19 vaccines have been developed worldwide, including protein subunit, recombinant, viral vector, RNA- and DNA-based, and sub-unit vaccines [ 19 ].
Up to now, several COVID-19 vaccines have been authorized or approved for use. WHO issued an emergency use authorization for the Pfizer COVID-19 vaccine On December 31, 2020 (BNT162b2). Next, on February 15, 2021, the Astra-Zeneca/Oxford COVID-19 vaccine (manufactured by the Serum Institute of India and SKBio) received emergency use approval, followed by Ad26.COV2.S (developed by Janssen (Johnson & Johnson)) on March 12, 2021, and Moderna vaccine on April 30, 2021 [ 20 ]. Pfizer COVID-19 vaccine is a lipid nanoparticle formulation that contains a nucleoside-modified RNA against the S protein of the SARS-CoV-2 virus [ 21 ]. Moderna is a lipid nanoparticle–encapsulated nucleoside-modified messenger RNA vaccine encoding prefusion stabilized full-length spike protein of SARS-CoV-2 (24). The Oxford/AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19 vaccine, AZD1222) contains a replication-deficient chimpanzee adenoviral vector ChAdOx1, delivering the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene (22, 23). Janssen is a non-replicating, recombinant human adenovirus type 26, containing a full-length SARS-CoV-2 S protein [ 22 ]. Bharat (CovaxinTM) is an inactivated-virus vaccine developed in Vero cells combined with Alhydroxiquim-II (Algel-IMDG), chemosorbed imidazoquinoline onto aluminum hydroxide gel. This complex is an adjuvant to boost immune response for longer-lasting immunity [ 23 ].
Careful planning for the COVID-19 vaccination program requires comprehensive review studies to evaluate the efficacy and safety of the vaccines. This study aims to conduct a meta-analysis to assess the Effectiveness and Efficacy of COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson. Well-designed meta-analysis studies will provide a more accurate overview to evaluate Efficacy and safety outcomes compared to individual studies and contribute to a better understanding of the use of the vaccine in different populations.
Materials and methods
The present systematic review and meta-analysis were conducted according to Preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines for reviewing analytical observational studies [ 24 ].
Search strategy and screening
International databases were searched to find all original published articles, including Medline (PubMed), Web of Science, Embase (Elsevier), Cochrane Library, Scopus, Ovid, and CINHAL, to retrieve all articles evaluating and reporting the efficacy and side effects of all COVID-19 vaccine (Pfizer–BioNTech, Oxford–AstraZeneca, Moderna, Janssen, CoronaVac, Covaxin, Novavax and Convidecia) in fully vaccinated and partially vaccinated people. The studies which have compared these items with non-vaccinated individuals were also included. In addition to searching the mentioned databases, gray literature was searched by reviewing articles in the first ten pages of Google scholar. A manual search was performed by reviewing references from related studies. This search was conducted with language limitations from November 2020 to September 2022. The search protocol was developed based on four primary roots involving “vaccination,“ “COVID-19,“ “Side effect,“ and “Efficacy.“ All related components to these keywords were “vaccinated”, “non-vaccinated”, “partial vaccinated”, “fully vaccinated”, “Pfizer–BioNTech”, “Oxford–AstraZeneca”, “Sinopharm BIBP”, “Moderna”, “Janssen”, “CoronaVac”, “Covaxin”, “Novavax”, “Convidecia”, “symptoms”, “signs” (“fever”, “cough”, “malaise”, “dyspnea”, “myalgia”, “sore throat”, and “diarrhea”), “thrombosis”, “emboli”, “thromboembolism”, “thromboembolic”, which were added to the searched queries based on scientific Mesh terms, EMTREE, and Thesaurus. Reference Manager bibliographic software was applied to manage searched citations. Duplicate entries were searched by considering the papers’ title, year of publication, authors, and specifications of types of sources. In case of questionable records, the texts were compared. After reviewing the primary search results, each article was double-checked by title and available abstract, and some of the articles were omitted based on the selection criteria. The evaluation of the considered papers was based on the inclusion and exclusion criteria by the two researchers separately (SM, MS). After the screening, (YM) selected the articles by evaluating their full texts.
Eligibility criteria
We included all observational and interventional studies that assessed the Efficacy/Effectiveness and side effects of all types of COVID-19 vaccines (Pfizer–BioNTech, Oxford–AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin, Novavax and Convidecia) in fully vaccinated and partially vaccinated people. The studies comparing these items with non-vaccinated individuals were also included. We excluded duplicate citations, non-peer-reviewed articles in which the abstract and full text were unavailable, and other languages.
Data extraction
After screening according to the three assessment steps for titles, abstracts, and full texts, the full text of each selected article was extracted for detailed analysis. The data were retrieved using a checklist recording author, publication year, type of study, mean age, sample size, number of positive tests, Effectiveness/Efficacy after one dose, Effectiveness/Efficacy after the second dose, and number of confirmed COVID cases, hospitalization, and death. From systematic search to final data extraction, all processes were followed independently by two research experts (PM, FM). After the screening, the data extraction was finally approved by (YM).
Risk of bias
The qualitative evaluation of studies was done according to the Newcastle-Ottawa Quality Assessment Scale (NOS) [ 25 ] by two of the authors (FM, YM). This scale is designed to evaluate the qualitative properties of observational studies (random clinical trials, case-control, retrospective, cohort, and cross-sectional studies). NOS examined each study through six items in three groups: selection, comparability, and exposure. Stars were given to each item, and the maximum score was 9. If the scores assigned to the published articles differed, the external discussion method would be used [ 26 , 27 ].
The Jadad checklist was used by two separate authors (PM and FM) to explore potential risks of bias in interventional studies. These scales include items to assess the adequacy of random sequence generation, allocation concealment, blinding, the detection of incomplete outcome data, selective outcome reporting, and other potential sources of bias [ 28 ].
Statistical analysis
The random-effects model was used to calculate the pooled Effectiveness/Efficacy with a 95% confidence interval (95% CI) with Metaprop order. Calculating the cumulative relative risk (RR) with the 95% confidence interval and the meta set command was used considering the relative risk’s logarithm and logarithm standard deviation. Statistical analysis was performed using STATA 16.0 (Stata Corp, College Station, TX, USA), and statistical significance was considered at P-Value < 0.05. Heterogeneity among studies was evaluated by applying the I square value and reported as a percentage (%) to show the extent of variation between studies. A forest plot was used for presenting the meta-analysis results schematically. Egger’s test and funnel plot were applied to evaluate the publication bias. In addition, a subgroup analysis was done to identify different sources of heterogeneity.
Results and discussion
Characteristics of included studies and the participants.
A total of 2622 publications were screened for evaluating two items about COVID-19 vaccines: (I) Efficacy and (II) Effectiveness. These two items were assessed according to the virus variant (Alpha, Beta, Delta, and Gamma) and the type of vaccine (AstraZeneca, Pfizer, Moderna, Janssen, and Bharat). Data on other vaccines were not included due to inadequate published data. Of these publications, 20 studies met the systematic reviews’ inclusion criteria (non-randomized and randomized) and were included in our meta-analysis (Fig. 1 ).
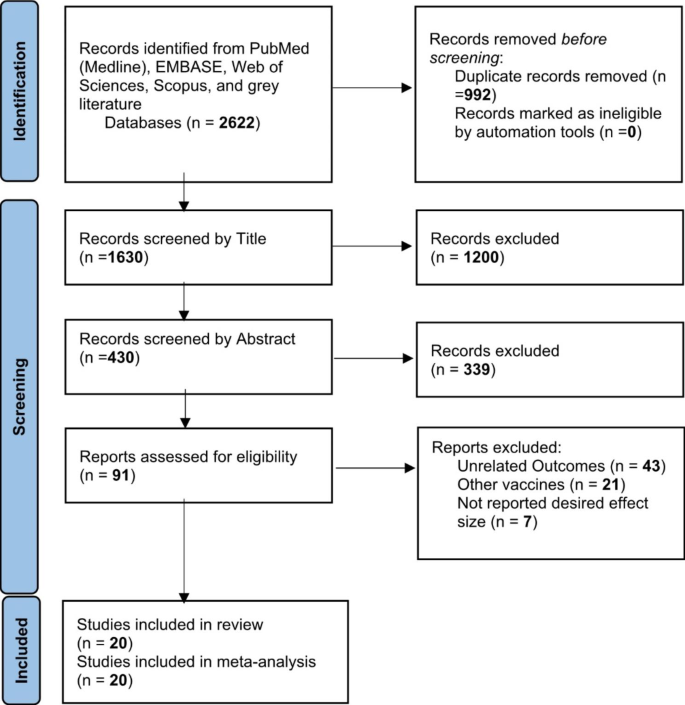
Identification of studies via databases and registers
One study was the cohort, four were randomized clinical trials (RCT), and fifteen were case-control. Clinical trials have evaluated vaccines’ efficacy, and observational studies such as cohorts and case controls have assessed their effectiveness. All selected papers were written in English. A total of 1,246,266 cases were included in this study that had received the COVID-19 vaccines. All vaccines were injected intramuscularly (IM). The participants were > 12 years old. The characteristics of included studies have been summarized in Table 1 .
The overall effectiveness of COVID-19 vaccines
After the first dose of the vaccine, the overall effectiveness of all COVID-19 vaccines was estimated to be 71% (95% CI 0.65, 0.78) (Fig. 2 ).
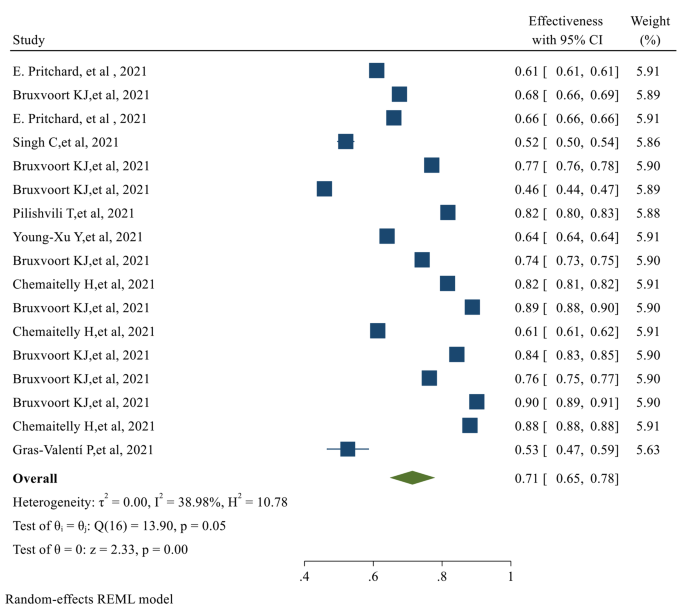
The overall Effectiveness of studied COVID-19 vaccines after the first dose
The overall Effectiveness of vaccines after the second dose was 91% (95% CI 0.88, 0.94), with a significant P-value ( p-value < 0.05 ) (Fig. 3 )
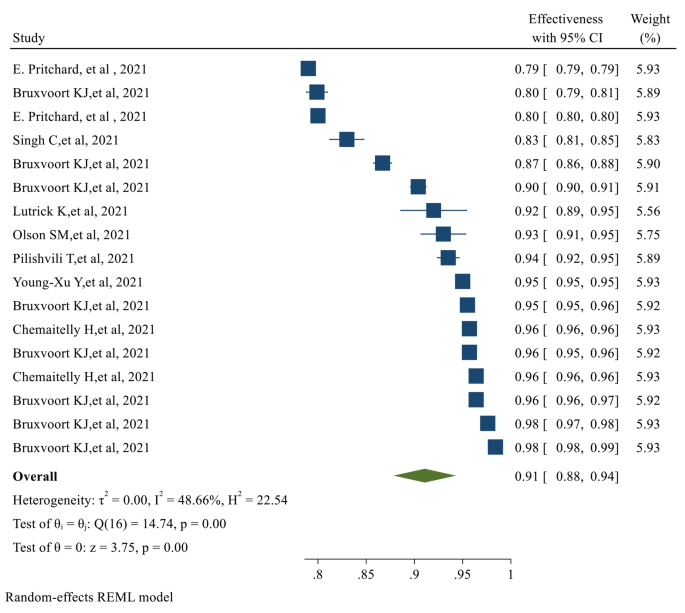
The overall Effectiveness of studied COVID-19 vaccines after the second dose. The overall Efficacy of COVID-19 vaccines
The overall Efficacy of the first dose of the vaccines evaluated in our study was 81% (95% CI 0.70, 0.91) (Fig. 4 )
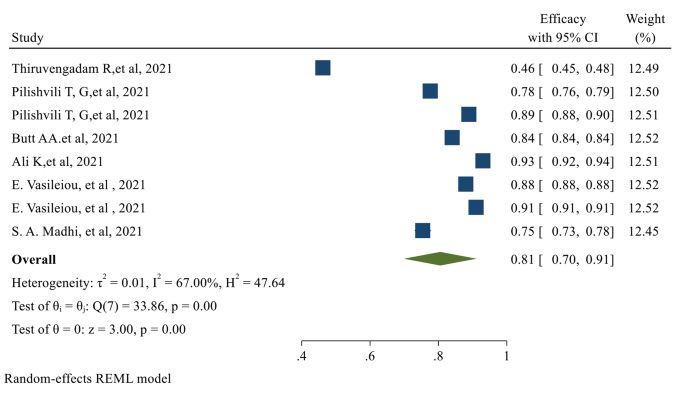
The overall Efficacy of the first dose of the studied vaccines
After the second dose of vaccination, the overall Efficacy of vaccines was 71% (95% CI 0.62, 0.79) with a significant P-value (Fig. 5 )
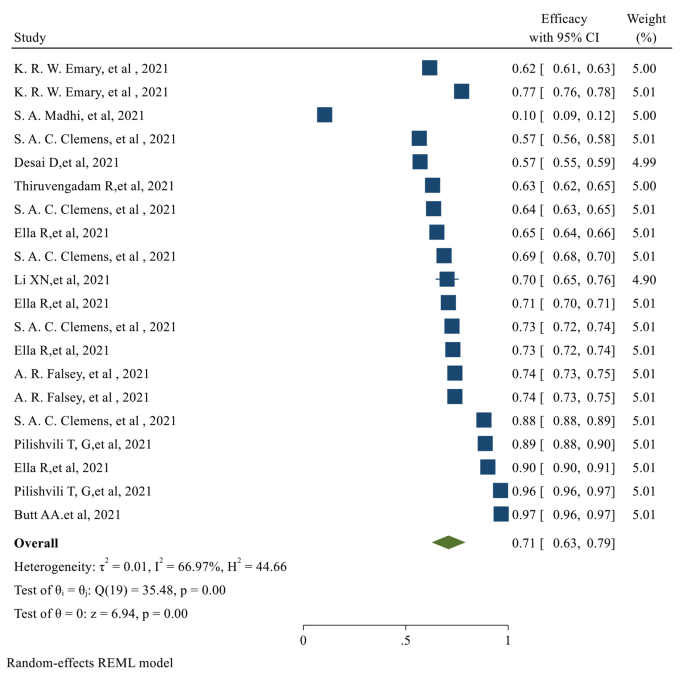
The overall Efficacy of the studied vaccines after the second dose
The individual efficacy of COVID-19 vaccines
The efficacy after the first dose was evaluated only in 8 of the selected studies, which assessed the efficacy of the AstraZeneca and Pfizer vaccines. No data was published on the efficacy after the first dose for Moderna, Johnson & Johnson, and Bharat. After the first dose of AstraZeneca and Pfizer vaccines, the pooled efficacy was 78% (95% CI 0.062, 0.95) and 84% (95% CI 0.77, 0.92), respectively. Of the selected publications, eighteen studies reported the efficacy after the second dose of vaccinations. The published data for the second dose Efficacy was only available for AstraZeneca, Pfizer, and Bharat vaccines. The second dose pooled Efficacy for AstraZeneca, Pfizer, and Bharat was 67% (95% CI 0.54, 0.80), 93% (95% CI 0.85, 1.00), and 71% (95% CI 0.61, 0.82) respectively (Table 2 ).
The individual effectiveness of COVID-19 vaccines
The first dose Effectiveness of the vaccines was evaluated in seventeen studies. For Moderna, AstraZeneca, and Pfizer, the pooled effectiveness after the first dose was 74% (95% CI 0.065, 0.83), 69% (95% CI 0.55, 0.82), and 67% (95% CI 0.51, 0.83) respectively. It was observed that the Effectiveness of Moderna after the first dose was higher than other types of vaccines. The second dose Effectiveness of the vaccines was reported in 17 studies. The pooled effectiveness after the second dose of Moderna, AstraZeneca, and Pfizer vaccines was 93% (95% CI 0.89, 0.97), 89% (0.80, 0.97), and 90% (95% CI 0.83, 0.96) respectively; Moderna had higher effectiveness after the second dose, among other studied vaccines (Table 2 ).
Efficacy of the vaccines against the virus variants
The overall first and second-dose vaccination Efficacy against different COVID-19 variants is listed in Table 2 . The first dose of overall vaccine Efficacy against the Alpha variant was 84%, which was higher than other variants (95% CI 0.84, 0.84). The overall efficacy of the first dose vaccination against the delta variant was only 46% (95% CI 0.45, 0.48), which was the lowest. Similarly, the highest second dose Efficacy was observed against the Alpha variant, which was 77% (95% CI 0.57, 0.97). The overall efficacy of the second dose against the Delta and Beta variants was 64% (95% CI 0.58, 0.69) and 10% (95% CI 0.09, 0.12), respectively.
Effectiveness of the vaccines against the virus variants
The overall first and second-dose vaccination Effectiveness against different COVID-19 variants is reported in Table 2 . The first dose Effectiveness of vaccination against the Gamma variant was 74% (95% CI 0.73, 0.75) which was more than other variants. However, the overall first dose Effectiveness was 82% (95% CI 0.81, 0.82). After the second dose, the highest effectiveness was against the Beta variant (96% (95% CI 0.96, 0.96)). The overall effectiveness after the second vaccination dose was 96% (95% CI 0.096, 0.96) (Table 2 ).
The risk of confirmed COVID infection after vaccination (risk ratio)
Two categories of the selected studies assessed the risk ratio of COVID after vaccination: observational and experimental. Only the pooled risk ratio of AstraZeneca was evaluated in the experimental studies, which was 50% (95% CI 0.35, 0.71). In the observational studies, AstraZeneca and Moderna had the lowest pooled risk ratios, which were 18% (95% CI 0.04, 0.84) and 19% (95% CI 0.17, 0.22), respectively. Bharat had the highest pooled risk ratio (82% (95% CI 0.75, 0.89) (Table 3 ); however, the number of studies on the Bharat vaccine was fewer than other types of vaccines. Based on the reported experimental studies for the vaccine variants, the Beta variant had the highest (79% (95% CI 0.43, 1.44)), and the Gamma variant had the lowest risk ratio (31% (95% CI 0.18, 0.54)). In the observational studies, Delta had the highest (52% (95% CI 0.27, 1.01), and Gamma had the lowest risk ratio (2% (95% CI 0.02, 0.02)) (Table 3 ).
Since the emergence of COVID-19, the effort to develop effective vaccines against the infection has been started. Due to the highly contagious nature of the virus, vaccination has been considered a significant measure in the fight against COVID-19. World Health Organization (WHO) allows countries to issue emergency use authorizations for COVID-19 vaccines in line with their national regulations and legislation. Domestic emergency use authorizations are issued at the countries’ discretion and are not subject to WHO approval. Up to now, several vaccines have been developed and marketed to limit the spread of COVID-19 infection. As of January 12, 2022, several COVID 19 vaccines have been given Emergency Use Listing (EUL), including those developed by Pfizer/BioNTech, AstraZeneca, Johnson & Johnson, Moderna, Sinopharm, Sinovac, Bharat Biotech, etc. [ 29 ].
Despite the significant role of COVID-19 vaccination in confining the infection, vaccines’ Efficacy and Effectiveness have not yet been comprehensively discussed. The present study meticulously looked into the Efficacy and Effectiveness of several vaccines.
Our analysis revealed that the overall effectiveness of the studied vaccines after the first dose is significantly less than their effectiveness after the second dose. The first dose’s effectiveness was evaluated in 17 studies. After the first dose, Moderna, AstraZeneca, and Pfizer’s Effectiveness was 74%, 69%, and 67%, respectively. The Effectiveness of Moderna after the first dose was higher than other types of studied vaccines. Second dose Effectiveness was evaluated in 17 studies. After the second dose of Moderna, AstraZeneca, and Pfizer vaccination, the effectiveness was 93%, 89%, and 90, respectively. Moderna provided higher effectiveness after the second dose among other studied vaccines. Therefore, administering the second dose should produce a more reliable response and higher effectiveness than a single dose.
Surprisingly, the overall efficacy of the first dose was significantly more than the second dose; 81% (95% CI 0.70, 0.91) for the first dose compared to 71% (95% CI 0.62, 0.79) for the second dose. This can be explained by the fact that the efficacy after the first dose was evaluated only in 8 studies that assessed only AstraZeneca and Pfizer vaccines. No data was available regarding the efficacy after the first dose of Moderna, Bharat, and Johnson & Johnson vaccines. We observed that the first dose Efficacy of the Pfizer vaccine is significantly more than the AstraZeneca vaccine. The Efficacy for AstraZeneca and Pfizer after the first dose vaccination was 78% and 84%, respectively. Concerning the second dose Efficacy, the published data were available only for AstraZeneca, Pfizer, and Bharat. In Total, eighteen studies evaluated the efficacy of these vaccines after the second dose. The Efficacy for AstraZeneca, Pfizer, and Bharat was 67%, 93%, and 71%, respectively.
We also investigated the Efficacy and Effectiveness of the first and second-dose vaccination against the COVID-19 virus variants. The overall efficacy of vaccination against the Alfa variant after the first dose was 84%, which was more than other variants. The highest efficacy after the second dose vaccination was also observed for the Alpha variant (77%). The first dose’s effectiveness against the Gamma variant was the highest (74%). Although, the overall first dose effectiveness was 82%. The highest second dose Effectiveness was against the Beta variant (96%), and the overall effectiveness after the second vaccination dose was 96% against all variants.
Up to now, there are other meta-analyses published on the efficacy and effectiveness of the COVID-19 vaccines. For example, in the meta-analysis reported by Pormohammad et., al, the efficacy of mRNA-based and adenovirus-vectored COVID-19 vaccines in phase II/III randomized clinical trial has been reported as 94.6% (95% CI 0.936–0.954) and 80.2% (95% CI 0.56–0.93), respectively. Additionally, the mRNA-based vaccines showed the highest reported side effects except for diarrhea and arthralgia [ 30 ]. However, the research had not reported the efficacy against different variants of the COVID-19 virus. Moreover, the Efficacy and Effectiveness of individual vaccines have not been mentioned; the vaccine Efficacy has been reported based on the vaccine classes. Another meta-analysis reported that the effectiveness of the Pfizer-BioNTech and Moderna vaccines was 91.2% and 98.1%, respectively, while the effectiveness of the CoronaVac vaccine was 65.7% in fully vaccinated individuals [ 31 ]. However, this study has not reported the effectiveness of the vaccines against COVID-19 variants or their efficacy.
Additionally, A previously reported network meta-analysis of various COVID-19 vaccines found Moderna was the most effective vaccine against COVID-19 infection, with an efficacy rate of 88%, followed by Sinopharm and Bharat. The least effective vaccines were Coronavac, Curevac, and AstraZeneca. The mRNA-based vaccines were superior in preventing infection and symptomatic infection, while the inactivated vaccines were most effective in preventing severe COVID-19 infection. Concerning safety, Sinopharm had the highest safety profile in local side effects, while ZF2001 had the highest safety in unsolicited side effects. Inactivated vaccines had the best safety profile in local and systemic side effects, while mRNA-based vaccines had the poorest safety profile. Thromboembolic events were reported after J&J, AstraZeneca, Pfizer, and Moderna vaccine administration. However, no confirmed vaccine-Induced Thrombotic Thrombocytopenia (VITT) cases were reported after mRNA vaccines [ 32 ].
It is necessary to mention that some vaccines’ overall or variant-specific Effectiveness and Efficacy are unavailable after the first or second dose. Moreover, the timing of the second dosing of the vaccines is not elicited in some trials, which may have led to the lower observed overall efficacy after the second dose. Additionally, some reports had noticeable bias by not including enough samples or not considering a broad enough geographical, economic, and age diversity.
We searched various databases and websites to include the maximum number of relevant publications to prevent database bias; after performing Egger’s regression test, we did not find significant publication bias. However, publication bias and heterogeneity for some pooled results must be considered when interpreting the outcomes.
Despite the valuable information provided by this meta-analysis, the study has some limitations to consider, such as the time frame of the studies (November 2020 to April 2022), the exclusion of unpublished data or ongoing investigations, the subjectivity of study selection criteria, and the limited number of vaccines evaluated. Additionally, the study did not consider differences in vaccine distribution among countries or provide data on the vaccines’ effectiveness against severe disease, hospitalization, or death. Despite its limitations, the meta-analysis highlights the need to continue monitoring the vaccines’ effectiveness.
In conclusion, Moderna, an mRNA-based vaccine, showed the highest total effectiveness after the first dose. Although the Pfizer vaccine showed a higher Efficacy after the first and second doses than AstraZeneca and Bharat, our conclusion has some limitations due to the lack of any published study regarding the Moderna and Johnson & Johnson vaccines’ efficacy. First-dose vaccination generally showed the highest overall effectiveness against the Gamma variant. Second dose vaccination showed a 96% overall Effectiveness against all variants. The efficacy of vaccination against the Alfa variant after the first dose was more than other variants. The highest efficacy after the second vaccination dose was also observed for the Alpha variant. Due to the timeline of the studies, all the vaccines are missing longer-term Efficacy and Effectiveness evaluations. This meta-analysis incorporated all relevant studies for summarizing and analyzing the Effectiveness and Efficacy of several vaccines for COVID-19. The results of this study support the overall Efficacy and Effectiveness of all studied COVID-19 vaccines and support the ongoing global public health effort for vaccination against COVID-19.
Data Availability
The data extracted for analyses are available by the corresponding author upon reasonable requests.
Abbreviations
Coronavirus Disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
World Health Organization
She J, et al. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020;9(1):19.
Article PubMed PubMed Central Google Scholar
Lu R, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Article CAS PubMed PubMed Central Google Scholar
Zhu N, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Gilzad-Kohan H, Jamali F. Anti-inflammatory Properties of drugs used to Control COVID-19 and their Effects on the renin-angiotensin system and angiotensin-converting Enzyme-2. J Pharm Pharm Sci. 2020;23:259–77.
Article PubMed Google Scholar
Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9.
Guan WJ, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Article CAS PubMed Google Scholar
Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Balasubramani J, Anbalagan M. Research productivity on COVID-19 in Dimension database: an analytical study. J Emerg Manag. 2021;18(7):63–9.
Chen T, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Islam MI. Current drugs with potential for treatment of COVID-19: a Literature Review. J Pharm Pharm Sci. 2020;23(1):58–64.
Article Google Scholar
Çalık Başaran N, et al. Outcome of noncritical COVID-19 patients with early hospitalization and early antiviral treatment outside the ICU. Turk J Med Sci. 2021;51(2):411–20.
Tsang HF, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877–88.
Singh AK, et al. Molnupiravir in COVID-19: a systematic review of literaturef. Diabetes Metab Syndr. 2021;15(6):102329.
WHO., WHO Coronavirus (COVID-19) Dashboard. 2021.
Checcucci E et al. The vaccine journey for COVID-19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med, 2020.
Rego GNA et al. Current clinical trials protocols and the global effort for immunization against SARS-CoV-2. Vaccines (Basel), 2020. 8(3).
Smit M, et al. Prophylaxis for COVID-19: a systematic review. Clin Microbiol Infect. 2021;27(4):532–7.
WHO., The COVID-19 vaccine tracker. 2021.
Francis AI, et al. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. 2022;98(1159):389–94.
Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine; 2020.
Sadoff J, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201.
Kumar VM, et al. Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. npj Vaccines. 2021;6(1):1–7.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Wells G et al. The Newcastle-Ottawa Quality Assessment Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Clin Epidemiol [Internet], 2017: p. 1–2.
Von Elm E, et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7.
Von Elm E, et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
Moher D, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73.
WHO. Coronavirus disease (COVID-19): Vaccines. 2022; Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines .
Pormohammad A et al. Efficacy and safety of COVID-19 vaccines: a systematic review and Meta-analysis of Randomized clinical trials. Vaccines (Basel), 2021. 9(5).
Zheng C, et al. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–60.
Toubasi AA, et al. Efficacy and safety of COVID-19 vaccines: a network meta-analysis. J Evid Based Med. 2022;15(3):245–62.
Download references
Acknowledgements
Not applicable.

Author information
Authors and affiliations.
Department of Pharmaceutical and Administrative Sciences, College of Pharmacy, Western New England University, 1215 Wilbraham Road, Springfield, MA, 01119, USA
Marzieh Soheili
Department of Physical Medicine and Rehabilitation, School of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
Sorour Khateri
Social Determinants of Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
Farhad Moradpour & Yousef Moradi
Department of Epidemiology and Biostatistics, School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
Pardis Mohammadzedeh & Mostafa Zareie
Pediatric Gastroenterology Fellowship, Department of Pediatrics, School of Medicine, Namazi teaching Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
Seyede Maryam Mahdavi Mortazavi
Massachusetts College of Pharmacy and Health Sciences (MCPHS), 179 Longwood Avenue, Boston, MA, 02115, USA
Sima Manifar
Hamed Gilzad Kohan
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: YM. Acquisition, analysis, and interpretation of data: YM, MS, HGK, FM, PM, SK, MZ, SM, and SMMM. Drafting of the manuscript: YM, HGK, MS. Critical revision of the manuscript for the important intellectual content: YM, MS. Project administration: YM and HGK. All authors have approved the final manuscript draft.
Corresponding authors
Correspondence to Hamed Gilzad Kohan or Yousef Moradi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Soheili, M., Khateri, S., Moradpour, F. et al. The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis. Ann Clin Microbiol Antimicrob 22 , 42 (2023). https://doi.org/10.1186/s12941-023-00594-y
Download citation
Received : 04 October 2022
Accepted : 10 May 2023
Published : 19 May 2023
DOI : https://doi.org/10.1186/s12941-023-00594-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
Annals of Clinical Microbiology and Antimicrobials
ISSN: 1476-0711
- Submission enquiries: [email protected]
Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
Affiliations.
- 1 Thibodaux Regional Health System, Thibodaux, LA, USA. Electronic address: [email protected].
- 2 Unit of Innovation and Organization, Navarre Health Service, Spain. Electronic address: [email protected].
- 3 Institute of Evidence-Based Healthcare, Bond University, Gold Coast, QLD, Australia. Electronic address: [email protected].
- 4 Fielding School of Public Health and College of Letters and Science, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 5 Geffen School of Medicine, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected].
- 7 School of Pharmacy, University of Maryland, Baltimore, MD, USA. Electronic address: [email protected].
- PMID: 36055877
- PMCID: PMC9428332
- DOI: 10.1016/j.vaccine.2022.08.036
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
Methods: Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults ( NCT04368728 and NCT04470427 ), focusing analysis on Brighton Collaboration adverse events of special interest.
Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2 (95 % CI -0.4 to 20.6 and -3.6 to 33.8), respectively. Combined, the mRNA vaccines were associated with an excess risk of serious adverse events of special interest of 12.5 per 10,000 vaccinated (95 % CI 2.1 to 22.9); risk ratio 1.43 (95 % CI 1.07 to 1.92). The Pfizer trial exhibited a 36 % higher risk of serious adverse events in the vaccine group; risk difference 18.0 per 10,000 vaccinated (95 % CI 1.2 to 34.9); risk ratio 1.36 (95 % CI 1.02 to 1.83). The Moderna trial exhibited a 6 % higher risk of serious adverse events in the vaccine group: risk difference 7.1 per 10,000 (95 % CI -23.2 to 37.4); risk ratio 1.06 (95 % CI 0.84 to 1.33). Combined, there was a 16 % higher risk of serious adverse events in mRNA vaccine recipients: risk difference 13.2 (95 % CI -3.2 to 29.6); risk ratio 1.16 (95 % CI 0.97 to 1.39).
Discussion: The excess risk of serious adverse events found in our study points to the need for formal harm-benefit analyses, particularly those that are stratified according to risk of serious COVID-19 outcomes. These analyses will require public release of participant level datasets.
Keywords: Adverse events of special interest; Brighton Collaboration; COVID-19; COVID-19 vaccines; Coalition for Epidemic Preparedness Innovations; Moderna COVID-19 vaccine mRNA-1273; NCT04368728 ; NCT04470427 ; Pfizer-BioNTech COVID-19 vaccine BNT162b2; SARS-CoV-2; Safety Platform for Emergency vACcines; Serious adverse events; Vaccines; mRNA vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Publication types
- Clinical Trial, Phase III
- Randomized Controlled Trial
- COVID-19 Vaccines / adverse effects
- COVID-19* / prevention & control
- RNA, Messenger
- Randomized Controlled Trials as Topic
- Vaccination / adverse effects
- Vaccines, Synthetic
- mRNA Vaccines
- COVID-19 Vaccines
Associated data
- ClinicalTrials.gov/NCT04368728
- ClinicalTrials.gov/NCT04470427
Economics CAGE Research Centre
The psychological gains from covid-19 vaccination: who benefits the most.
594/2021 Manuel Bagues and Velichka Dimitrova
We quantify the impact of COVID-19 vaccination on psychological well-being using information from a large-scale panel survey representative of the UK population. Exploiting exogenous variation in the timing of vaccinations, we find that vaccination increases psychological well-being by 0.12 standard deviation, compensating for around one half of the overall decrease caused by the pandemic. This effect persists for at least two months, and it is associated with a decrease in the perceived likelihood of contracting COVID-19 and higher engagement in social activities. The improvement is 1.5 times larger for mentally distressed individuals, supporting the prioritisation of this group in vaccination roll-outs.
Public Policy
Share Publication
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Inside the story about the research and development of COVID-19 vaccines
Shrina p patel, gayatri s patel, jalpa v suthar.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Jalpa V. Suthar, PhD. Department of Pharmacology& Clinical Pharmacy, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology (Charusat Campus), Changa, Gujarat, India. Tel: +91-9825907538, Fax: +91-2697-247100, [email protected]
Corresponding author.
Received 2021 Mar 1; Accepted 2021 May 3; Issue date 2021 May.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/4.0/ ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The ongoing coronavirus threat from China has spread rapidly to other nations and has been declared a global health emergency by the World Health Organization (WHO). The pandemic has resulted in over half of the world's population living under conditions of lockdown. Several academic institutions and pharmaceutical companies that are in different stages of development have plunged into the vaccine development race against coronavirus disease 2019 (COVID-19). The demand for immediate therapy and potential prevention of COVID-19 is growing with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics. The challenging part is a need for vigorous testing for immunogenicity, safety, efficacy, and level of protection conferred in the hosts for the vaccines. As the world responds to the COVID-19 pandemic, we face the challenge of an overabundance of information related to the virus. Inaccurate information and myths spread widely and at speed, making it more difficult for the public to identify verified facts and advice from trusted sources, such as their local health authority or WHO. This review focuses on types of vaccine candidates against COVID-19 in clinical as well as in the preclinical development platform.
Keywords: COVID-19, Vaccine, SARS-CoV-2, Clinical trials, Vaccine development, Vaccine candidate
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originated in Hubei Province, China, in December 2019 (and possibly earlier, though unrecognized), as a pneumonia-causing disorder [ 1 ], most likely the result of natural selection in animal hosts (bats, pangolins) before the zoonotic transition [ 2 ]. Seven members of this viral family are now known to infect humans, three of whom have the potential to cause severe respiratory diseases [ 3 ]. Coronaviruses (CoVs) are positive-sense, single-stranded Coronaviridae family (subfamily Coronavirinae) RNA viruses that infect a broad range of hosts to produce diseases ranging from the common cold to severe/fatal diseases [ 4 ]. The novel virus was initially named “2019-nCoV” by the International Committee on Virus Taxonomy. It was changed to “SARS-CoV-2” since it was found to be the sister virus of an extreme acute respiratory syndrome (SARS-CoV) [ 5 ]. The ongoing threat of coronavirus emerging in China has spread rapidly to other countries and has been declared by the World Health Organization (WHO) as a global health emergency [ 6 ].
Virus genetic sequencing shows that it is a beta coronavirus that is closely related to the SARS virus [ 7 ]. Currently, immunization prevents 2–3 million deaths from more than 20 life-threatening diseases that are now being controlled by vaccinations, and work is underway at an unprecedented pace to make coronavirus disease 2019 (COVID-19) a vaccine-preventable illness [ 8 ]. To accelerate the research and development process and to establish new standards and standards to prevent the spread of the coronavirus pandemic and care for those affected, WHO brings together the world's scientists and public health practitioners [ 7 ]. In human medical intervention, vaccines are one of the monumental achievements in mitigating the dispersion and effects of infectious diseases [ 9 ]. Vaccines are the most useful method for contagious disease prevention because they are more cost-effective than treatment and reduce morbidity and mortality without long-lasting effects [ 10 ]. Preventive and therapeutic vaccines will be of fundamental significance as the most obvious way to safeguard public health [ 11 ]. Since the coronavirus shares substantial sequence homology with two other lethal coronaviruses, SARS and Middle East respiratory syndrome (MERS), the vaccines identified could potentially promote the design of anti-SARS-CoV-2 vaccines. It is essential to establish safe and effective vaccines to contain the COVID-19 pandemic, eradicate its spread, and eventually prevent its future recurrence [ 12 ]. By exposing individuals to antigens, vaccination can produce long-lasting immunity to drive the production of immunological memory before meeting live pathogens. Thus the resulting immunity can be mediated by the activation of humoral antibodies and the effector function of cellular T-cells [ 13 ]. The full development path for an effective SARS-CoV-2 vaccine will involve th e cooperation of industry, government, and academia in unprecedented ways, each contributing its strengths [ 14 ].
It is a difficult task to develop a SARS-CoV-2 vaccine to control its spread and help remove it from the human population since there is a lack of knowledge on its biological properties, epidemiology, individual immune responses to it, and so forth [ 15 ]. The S protein is the critical target of vaccine production since it includes a receptor-binding domain (RBD) and viral functions. It will be essential to confirm the clinical significance of the SARS-CoV-2 binding and neutralizing antibody titers and their ability to predict efficacy [ 16 ]. Only in a significant clinical efficacy study would it be possible to confirm the association between antibody titers and defense against COVID-19 [ 17 ]. For any frequently used vaccine, there is a theoretical risk that vaccination could cause subsequent infection with SARS-CoV-2 more severe. This has been confirmed in feline coronaviruses and has been observed in some SARS-CoV-1 animal vaccine challenge models [ 18 ].
The key benefit of next-generation vaccines is that they can be produced based on sequence data alone [ 19 ]. If the viral protein(s) that are essential for the defense against infection or disease and therefore for inclusion in the vaccine is established, the availability of coding sequences for the viral protein(s) is sufficient to start the production of the vaccine rather than to rely on the ability to grow the virus [ 20 ]. This makes these platforms extremely adaptable and dramatically accelerates the production of vaccines, as is evident from the fact that the majority of currently underway clinical trials of COVID-19 vaccines include a next-generation platform [ 19 ]. A prospective pharmaceutical manufacturer must send an application to a regulatory authority such as the Food and Drug Administration (FDA) to examine the new vaccine after a possible vaccine has been announced by a researcher [ 21 ].
The demand for immediate therapy and potential prevention of COVID-19 is growing [ 22 ] with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics [ 23 ]. Attempts are being made to establish secure and successful methods for prophylactics [ 24 , 25 ]. Several vaccines are in different phases of clinical trials [ 6 ], but there is a lack of prophylactics in the present scenario [ 26 ]. Several attempts have been made to create COVID-19 vaccines to avoid the pandemic condition as well as the S-protein SARS-CoV-2 has been used for most of the emerging vaccine candidates. In Fig. 1 , the overview of vaccine candidates in their respective ongoing clinical phases depicts the percentage of vaccine candidates amongst which the majority of developing vaccines is in phase 1/2. The data shown below in the graph is assessed until 15 October 2020, in the pipeline of vaccine development and registered clinical trials globally.
Fig. 1. Overview of vaccine candidates in their respective ongoing clinical phases.
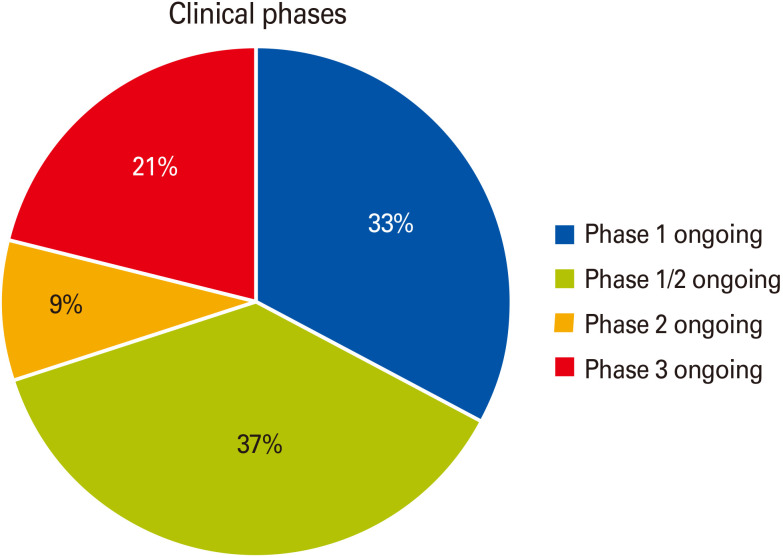
In Fig. 2 , the overview of the global COVID-19 vaccine landscape in clinical development depicts that there are seven major types of vaccine candidates for COVID-19 is illustrated as (inactivated, non-replicating viral vectors, replicating viral vectors, protein subunit, nucleic acid-based, and virus-like particles [VLP]), showing the percentage of candidate vaccines that are currently under clinical development. The nucleic acid-based platform includes both RNA vaccines and DNA vaccines. Among the seven types of vaccine candidates, protein subunit-based vaccines constitute the highest 31% in clinical development. In contrast, VLP-based vaccine and replicating viral vectors comprises the lowest as 5% in the clinical development.
Fig. 2. The overview of global coronavirus disease 2019 vaccine landscape in clinical development. VLP, virus-like particle.
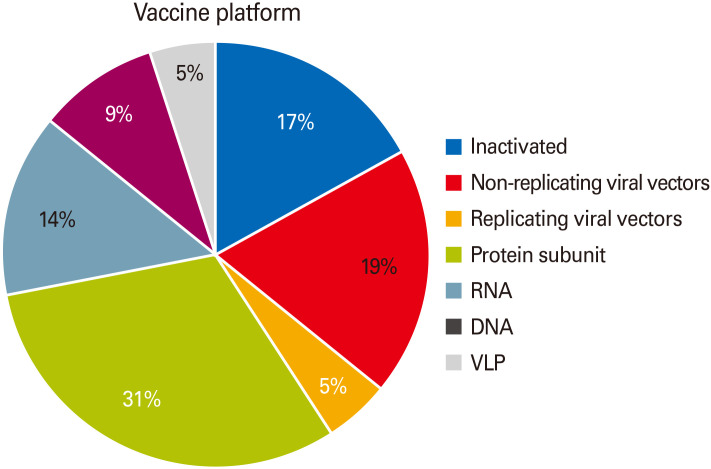
In Fig. 3 , the overview of global COVID-19 vaccine landscape in preclinical development depicts that there are 10 significant types of vaccine candidates for COVID-19 is illustrated as (inactivated, replicating bacteria vector, DNA, live attenuated virus, non-replicating viral vectors, protein subunit, t-cell based, replicating viral vectors, RNA, and VLP), showing the percentage of candidate vaccines that are currently under preclinical development. Among the 10 types of vaccine candidates, protein subunit-based vaccines constitute the highest 36% in clinical development whereas T-cell based vaccine and replicating bacteria vector comprises the lowest at 1% in the preclinical development globally.
Fig. 3. The overview of global coronavirus disease 2019 vaccine landscape in preclinical development. VLP, virus-like particle.
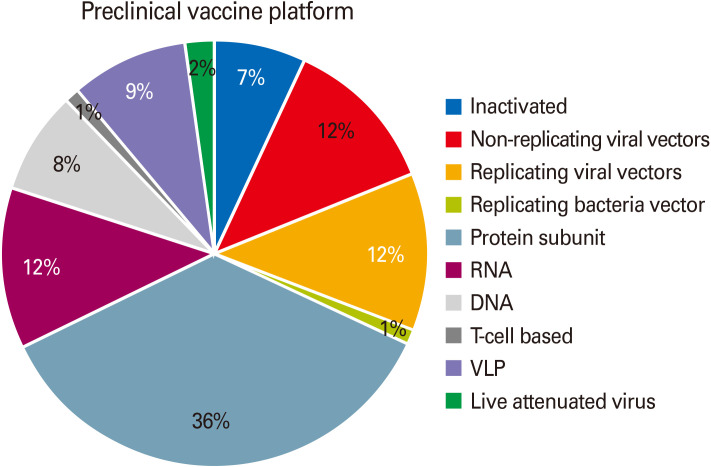
RNA-Based Vaccine
As a result of considerable developments in biotechnology, due to their higher potency, short development cycles, low-cost product, and safe administration, mRNA vaccines represent a substantial improvement over traditional vaccine strategies [ 27 ]. The mRNA is an evolving platform that is non-infectious and non-integrated and has almost no possible risk of insertional mutagenesis. Antigen discovery, sequence analysis, and optimization, screening of modified nucleotides, delivery system discovery, and immune response and safety assessment tests are the sequential events in the mRNA vaccine production process [ 28 ]. In vaccines, two primary forms of RNA are investigated: virally derived, RNA self-replicating, and mRNA non-replicating. The antigen and the necessary viral replication machinery are typically self-replicating RNAs, whereas conventional mRNA-based vaccines encode only the antigen of interest with 50 and 30 untranslated regions (UTRs) [ 27 ].
The immunogenicity of mRNA can be decreased, and changes can be made to enhance the stability of these vaccines [ 29 ]. Furthermore, anti-vector immunity is also resisted as mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine [ 30 ]. This platform has empowered the rapid vaccine development program due to its flexibility and ability to reproduce the structure and expression of the antigen as seen in the course of natural infection [ 31 ]. A possible benefit of mRNA vaccines is the convenient availability of a portable mRNA “printing” facility for large-scale production of mRNA [ 32 ].
mRNA-1273 (Moderna TX Inc.)
It is a vaccine composed of lipid nanoparticle (LNP) encapsulated synthetic mRNA that codes for SARS-CoV-2 full-length, pre-fusion stabilized spike protein (S) [ 33 ]. It has the potential to induce an antiviral response that is highly S-protein specific. Also, it is known to be relatively harmless since it is neither composed of the inactivated pathogen nor of the live pathogen sub-units [ 34 ]. To perform the phase II trials, the vaccine has received FDA fast-track approval. The company published the interim antibody data for phase I of eight participants who received different levels of dose [ 33 ]. For the participants receiving 100 µg dose, neutralizing antibody levels were significantly higher than those observed in convalescent sera. In the 25 µg and 100 µg dose cohorts, the vaccine was found to be primarily safe and well-tolerated. In comparison, three participants reported systemic symptoms of grade 3 following administration of the second 250 µg dose level [ 26 ]. The possible benefits of a prophylactic vaccine mRNA strategy include the ability to replicate natural infection to induce a more effective immune response and the ability to incorporate multiple mRNAs into a single vaccine [ 12 ].
On 24 February 2020, Moderna declared that it had released the first batch of mRNA-1273 against SARS-CoV-2 for human use, prepared using the methods and strategies outlined in its previous patents. mRNA-1273 vials have been shipped to the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH), to be used in the United States in the proposed phase 1 study [ 35 ]. In collaboration with researchers at the NIAID Vaccine Research Centre, Moderna reports that mRNA-1273 is an mRNA vaccine targeting a prefusion stabilized form of the S protein associated with SARS-CoV-2, which was chosen by Moderna [ 32 ]. Patent application WO2018115527 describes vaccines consisting of mRNA encoding at least one MERS coronavirus antigen, preferably an S protein or an S protein fragment (S1), an envelope protein (E), a membrane protein (M), or a nucleocapsid protein (N), all of which have been successful in inducing an immune response unique to the antigen [ 33 ]. Intradermal administration of a LNP-encapsulated mRNA mixture encoding MERS-CoV S proteins into mice has been shown to result in vivo translation and humoral immune response induction [ 12 ].
BNT162b1 (BioNTech, Fosun Pharma, Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that codes for the essential target of the neutralizing antibody virus, trimerized SARS-CoV-2 RBD [ 29 ]. The vaccine shows improved immunogenicity due to the addition of the foldon trimerization domain of T4 fibrin-derived to the RBD antigen. In 80 nm ionizable cationic LNPs, the mRNA is encapsulated, which guarantees its efficient delivery [ 31 ]. In phase 1/2 clinical trials, elevated levels of RBD-specific immunoglobulin G (IgG) antibodies with a geometric mean concentration were found to be 8 to 46.3 times the convalescent serum titer. Whereas, the SARS-CoV-2 neutralizing antibody geometric mean titers were found to be 1.8 to 2.8 times the convalescent serum panel [ 29 ]. With no adverse effects, mild and transient local reactions and systemic events were observed. The data review did not, however, assess the protection and immune response beyond 2 weeks after the second dose administration [ 31 ].
Report of available effectiveness, tolerability, and immunogenicity results from an ongoing placebo-controlled, observer-blinded dose-escalation study in healthy adults 18–55 years of age, randomized to receive two 21-day separate doses of 10 µg, 30 µg, or 100 µg of BNT162b1, a nucleoside-modified LNP mRNA vaccine encoding trimerized SARS-CoV-2 spike glycoprotein dose-dependent, usually mild to moderate, and temporary, was the local reactions and systemic events [ 29 ]. The BNT162b1 vaccine candidate now being clinically studied integrates such nucleoside modified RNA and encodes the SARS-CoV-2 spike protein RBD, a primary target of virus-neutralizing antibodies [ 31 ]. Sera's RBD-binding IgG and SARS-CoV-2 neutralizing titers increased both at the dose level and after the second dose. Geometric mean neutralizing titers were 1.8 to 2.8 times those of a panel of human sera convalescent COVID-19. These findings help further evaluation of this candidate for the mRNA vaccine [ 33 ]. By adding a T4 fibritin-derived “foldon” trimerization domain, the RBD antigen expressed by BNT162b1 is modified to improve its immunogenicity by a multivalent display. The RNA vaccine is formulated in LNPs for more effective delivery to cells after intramuscular injection [ 31 ]. In Table 1 , potential RNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ].
Table 1. Potential RNA-based vaccine candidates for COVID-19 in the clinical development pipeline.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LNP, lipid nanoparticle; NIAID, National Institute of Allergy and Infectious Diseases; P/B, prime/boost; RBD, receptor-binding domain.
Viral Vector-Based Vaccines
Viral vector-based vaccines have a high degree of protein expression and long-term stability, inducing strong immune responses [ 46 ]. These include vaccines focused on chemically weakened viruses used to bear antigens or pathogens of concern for immune response induction [ 47 ]. A possible prophylactic strategy against a pathogen is a viral vector-based vaccine. These vaccines are highly selective in transmitting genes to the target cells, are highly effective in gene transduction, and are useful in inducing immune responses [ 48 ]. They have a long-term and high level of antigenic protein expression and thus have an excellent potential for prophylactic use as these vaccines activate and facilitate cytotoxic T cells, eventually contributing to the elimination of infected virus cells [ 46 ]. The generation of immunity to the vector is an essential consideration for the development of virus vectored vaccines, which could impede the antigen-specific response to boost vaccination [ 49 ]. Reports from preclinical and clinical trials suggested that adequate safety can be obtained from a single dose [ 50 ].
Ad5-nCoV (CanSino Biologics Inc., Beijing Institute of Biotechnology)
A four-fold increase in RBD and S protein-specific neutralizing antibodies was observed within 14 days [ 51 ]. Ad5-nCoV is a recombinant type-5 adenovirus (Ad5) replication-defective vector expressing the recombinant SARS-CoV-2 spike protein. It was prepared by cloning, together with the plasminogen activator signal peptide gene, an optimized full-length gene of the S protein in the Ad5 vector devoid of genes E1 and E3 [ 29 ]. The vaccine was constructed from the Microbix Biosystem using the Admax system. A positive antibody reaction or seroconversion of immunization was identified in phase I clinical trials and peaked at day 28, post-vaccination. Also, the response of CD4+T cells and CD8+T cells peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity has partially restricted the reaction of both the antibody and the T cell [ 51 ]. The study would further assess the antibody response in recipients between 18 and 60 years of age who received one of three doses in the study, with follow-up at 3- and 6-months post-vaccination [ 29 ].
Coroflu (University of Wisconsin-Madison, FluGen, Bharat Biotech)
M2SR, a self-limiting variant of the influenza virus that is modified by spike protein sequence insertion of the SARS-CoV-2 gene. Besides, the vaccine expresses the influenza virus' hemagglutinin protein, thereby triggering an immune response to both viruses [ 52 ]. The M2SR is self-limiting and, since it lacks the M2 gene, does not undergo replication. It is capable of entering the cell, thereby causing immunity to the virus [ 32 ]. It is delivered intra-nasally, mimicking the normal viral infection pathway. Compared to intramuscular injections, this route stimulates many immune system modes and has higher immunogenicity [ 52 ].
LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
By using SMENP minigenes to engineer dendritic cells (DC) with a lentiviral vector expressing the conserved domains of the structural proteins SARS-CoV-2 and protease [ 29 ], the LV-SMENP-DC vaccine is prepared. Subcutaneous vaccine inoculation introduces antigen-presenting cell antigens, which eventually cause cytotoxic T cells and produce an immune response [ 48 ].
ChAdOx1 (University of Oxford)
The recombinant adenovirus vaccine ChAdOx1 was developed using codon-optimized S glycoprotein and synthesized at the 5 ends with the leading tissue plasminogen activator (tPA) sequence [ 50 ]. The SARS-CoV-2 amino acid coding sequence (2 to 1273) and the tPA leader have been propagated in the shuttle plasmid. This shuttle plasmid is responsible for the coding between the Gateway recombination cloning site of the main immediate-early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator sites and polyadenylation signal from bovine growth hormone (BGH) [ 29 ]. In the bacterial artificial chromosome, the adenovirus vector genome is built by inserting the SARS-CoV-2 S gene into the ChAdOx1 adenovirus genome's E1 locus. In the T-Rex human embryonic kidney 293 (HEK-293) cell lines, the virus was then allowed to replicate and purified by ultracentrifugation of the CsCl gradient [ 53 ]. The absence of any subgenomic RNA from preclinical trials in intra-muscularly vaccinated animals is suggestive of improved immunity to the virus [ 50 ]. Previous studies have proposed that the immune response should be marshalled by a single shot [ 53 ]. In Table 2 , potential viral vector-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ].
Table 2. Potential viral vector-based vaccine candidates for COVID-19 in the clinical development pipeline.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain; MVA, modified vaccinia Ankara.
Protein Subunit-Based Vaccines
Subunit vaccines, safer and more straightforward to manufacture, present a host with high immunogenicity with one or few antigens, but need adjuvants to evoke a strong defensive immune response [ 62 ]. A subunit vaccine is a synthetic peptide or recombinant antigenic protein-dependent vaccine which is essential for long-term protection and a therapeutic invigoration of the immune response [ 63 ]. The subunit vaccine exhibits low immunogenicity and requires an adjuvant's additional assistance to potentiate the vaccine-induced immune responses. An adjuvant may improve the biological half-life of the antigenic material, or the immunomodulatory cytokine response may be improved. The use of an adjuvant, therefore, helps to overcome the shortcomings of the protein subunit vaccines [ 64 ]. Subunit vaccines may be designed to concentrate the immune response on the neutralization of epitopes, thus preventing the development of non-neutralizing antibodies that may encourage disease-related antibody-dependent enhancement [ 65 ]. Antigenic proteins thought to cause a defensive immune response are used in protein subunit vaccines. The S protein of SARS-CoV-2 is the most appropriate antigen to induce neutralizing antibodies against the pathogen [ 13 ]. The S protein is comprised of two subunits. In the S1 subunit, the N-terminal domain, RBD, and receptor-binding motif (RBM) domains are found, while the S2 subunit consists of FP, HR 1, and 2 [ 62 ]. The virus reaches the cell by endocytosis using S-protein mediated binding to the human angiotensin-converting enzyme 2 (hACE2) receptor. Therefore, S-protein and its antigenic fragments are key objectives for the establishment of a subunit vaccine [ 63 ]. A complex protein with two conformation states, i.e., a pre-fusion and post-fusion state, is the S glycoprotein [ 62 ]. Therefore, the antigen must maintain its surface chemistry and the profile of the initial pre-fusion spike protein to retain the epitopes for igniting good quality antibody responses. Also, targeting the masked RBM as an antigen, it will increase the neutralizing antibody response and enhance the overall efficacy of the vaccine [ 66 ].
NVX-CoV2373 (Novavax Inc., Emergent BioSolutions)
NVX-CoV2373 is a nano-particle-mediated immunogenic vaccine-mediated on coronavirus S-protein, the recombinant expression of stable pre-fusion [ 67 ]. In the baculovirus system, the protein has been stably expressed. By inducing high levels of neutralizing antibodies, the company aims to use the matrix-M adjuvant to strengthen the immune response against the SARS-CoV-2 spike protein [ 35 ]. A single immunization in animal models resulted in a high level of anti-spike protein antibodies that blocked the binding domain of the hACE2 receptor and could elicit SARS-CoV-2 wild-type virus-neutralizing antibodies [ 68 ].
Molecular clamp stabilized spike protein vaccine candidate
It is being developed in partnership with GSK and Dynavax by the University of Queensland [ 29 ]. The University will have access to the vaccine adjuvant (AS03 Adjuvant) platform technology, which is believed to enhance the response of the vaccine and reduce the amount of vaccine needed per dose [ 69 ]. The University is developing a stabilized pre-fusion, recombinant viral protein subunit vaccine based on the molecular clamp technology. It has been established that this technology induces the development of neutralizing antibodies [ 34 ].
PittCoVacc (University of Pittsburgh)
It is a recombinant SARS-CoV-2 vaccine based on the micro-needle array (MNA) that involves administering rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens) [ 70 ]. A significant increase in statistically significant antigen-specific antibodies was found in the mice models in preclinical studies at the end of 2 weeks [ 29 ]. Furthermore, even after sterilization using gamma rays, the immunogenicity of the vaccine was maintained. Statistically, relevant antibody titers confirm the feasibility of the MNA-SARS-CoV-2 vaccine at the early stage and even before boosting [ 70 ].
Triple antigen vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype in which an engineered Saccharomyces cerevisiae expression platform (D-CryptTM) co-expresses the recombinant spike, membrane, and envelope protein of SARS-CoV-2 [ 71 ]. The proteins then, like the VLP, undergo self-assembly. The biophysical characterization of the VLP was simultaneously given by the transmission electron microscopy and allied analytical data [ 29 ]. After more research and development, this prototype has the potential to engage in preclinical trials as a vaccine candidate. Besides, cost-effectively, it is assumed to be safe and easy to produce on a mass scale [ 71 ]. In Table 3 , potential protein subunit-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
Table 3. Potential protein subunit-based vaccine candidates for COVID-19 in the clinical development pipeline.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HIV, human immunodeficiency virus; RBD, receptor-binding domain; FIH, first-in-human; FDA, U.S. Food and Drug Administration.
DNA-Based Vaccines
A typical DNA vaccine is a plasmid DNA molecule that codes for the host immune system to be presented with one or more antigens [ 62 ]. They have the advantages of stability and successful delivery over mRNA vaccines [ 84 ]. Still, since they are needed to reach the nucleus, they have the risk of vector mutations and incorporation into the host genome [ 85 ]. DNA vaccines reflect a revolutionary approach, followed by a wide variety of immune responses, by the direct injection of plasmids encoding antigens [ 86 ]. The most groundbreaking approach to vaccination is the introduction of the DNA vaccine that codes for the antigen and an adjuvant that stimulates the adaptive immune response [ 87 ]. The transfected cells express the transgene, which gives a steady supply of transgene-specific proteins very similar to the live virus [ 84 ]. Also, immature DCs, which eventually present the antigen on the cell surface to the CD4 + and CD8 + T cells in combination with the major histocompatibility complex (MHC) 2 and MHC 1 antigens, endocytose the antigen material, thereby stimulating both successful humoral and cell-mediated immune systems [ 87 ]. DNA vaccines are considered safe and stable and can be developed easily, but their immunogenicity and immune response efficiency in humans have not yet been demonstrated [ 21 ].
INO-4800 (Inovio Pharmaceuticals)
It is an anti-SARS-CoV-2 prophylactic DNA vaccine. It uses the SARS-CoV-2 codon-optimized S protein sequence to which an immunoglobulin E (IgE) leader sequence is attached [ 29 ]. Using BamHI and XhoI, the SARS-CoV-2 IgE-spike sequence was synthesized and digested. Under the management of IE CMV, and BGH polyadenylation signal, the digested DNA was incorporated into the expression plasmid pGX0001 [ 85 ]. In preclinical studies, the existence of functional antibodies and the response of T cells indicate that the vaccine will produce an efficient immune response within seven days after vaccination [ 88 ]. The vaccine has entered phase I clinical trials (phase I: NCT04336410 ) and it is anticipated that this phase of clinical trials will be completed by July, with participants receiving 1.0 mg of INO-4800 by electroporation with CELLECTRA 2000 per dosing visit. The research will assess the immunological profile, efficacy, and tolerability of the candidate vaccine in healthy human adults upon intradermal injection and electroporation [ 29 ]. INO-4800 and the previous Inovio vaccine INO-4700 express either SARS-CoV-2-S or MERS-CoV-S inside the same DNA vector, respectively [ 85 ]. The vaccine is delivered by intramuscular injection, accompanied by injection site electroporation. The need for electroporation could restrict INO-4800's ability to be expanded to the scales necessary for a global pandemic and may be difficult to handle globally [ 13 ].
bacTRL (Symvivo Corporation)
Symvivo Corporation's bacTRL platform uses the engineered probiotic Bifidobacterium longum to deliver a SARS-CoV-2-S expressing DNA vaccine into intestinal cells. The first-in-man study of the bacTRL platform will also be a phase I study of the COVID-19 vaccine, so no prior immunological results are available [ 13 ]. In Table 4 , DNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 89 , 90 , 91 , 92 , 93 , 94 ].
Table 4. Potential DNA-based vaccine candidates for COVID-19 in the clinical development pipeline.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Virus-Like Particles Vaccine
VLPs are particles that form spontaneously, consisting of many co-expressed or mixed structural viral proteins. Several commercial vaccines are based on VLPs, such as hepatitis B and human papillomavirus vaccines [ 95 ]. Without the need for adjuvants, these vaccines can be constructed and used. Only when antigens with neutralizing epitopes are extensively investigated is the production of such vaccines possible [ 22 ]. A VLP is a self-assembled nanostructure incorporating essential viral structural proteins. VLP is similar to true viruses' molecular and morphological features but is non-infectious and non-replicating due to the absence of genetic materials [ 26 ]. Successful applications of VLP have been proved by vaccinological and virological study [ 95 ]. In the ongoing battle against the COVID-19 pandemic, the development of SARS-CoV-2 VLPs is highly in demand as an accessibly safe and relevant substitute for naturally pathogenic viruses [ 26 ]. A study suggested the possible use of plant biotechnology for the development of low-cost COVID-19 vaccines and plant-made antibodies for diagnosis, prophylaxis, and therapy [ 22 ].
In the current research, we have established SARS-CoV-2 VLPs effectively, using the mammalian expression system [ 47 ], which helps maintain specific patterns of protein glycosylation [ 22 ]. For the efficient formation and release of SARS-CoV2 VLPs among the four SARS-CoV-2 structural proteins, we have shown that membrane protein (M) expression and small envelope protein (E) are essential [ 47 ]. Also, the corona-like structure presented in SARS-CoV-2 VLPs from Vero E6 cells is more stable and unified in comparison with those from HEK-293 T cells. Our data show that the molecular and morphological characteristics of native virion particles in SARS-CoV-2 VLPs make SARS-CoV-2 VLPs a promising candidate vaccine and a powerful tool for research into SARS-CoV-2 [ 96 ]. The immunogenic composition composed of MERS-CoV nanoparticle VLPs containing at least one trimer of S protein formed by baculovirus overexpression in Sf9 cells was disclosed in patent application WO2015042373 by Novavax in 2015 [ 35 ]. When administered along with their patented adjuvant Matrix M, this VLP preparation induced a neutralizing antibody response in mice and transgenic cattle. Sera preparations from vaccinated cattle (SAB-300 or SAB-301) were also injected into Ad5-hDPP4 transduced BALB/c mice before the MERS-CoV challenge [ 22 ]. With a single prophylactic injection, both SAB-300 and SAB-301 were able to protect these mice from MERS-CoV infection [ 96 ]. On 26 February, Novavax announced that due to their prior experience dealing with other coronaviruses, including both MERS and SARS, animal testing of possible COVID-19 vaccine candidates had begun. Using their recombinant nanoparticle vaccine technology along with their proprietary adjuvant matrix-M, their COVID-19 candidate vaccines targeting the S protein of SARS-CoV-2 were created [ 35 ].
UMass Medical School researchers have developed a framework to create vaccines using VLPs, which one scientist claims may be a successful and safer alternative to a COVID-19 vaccine. Trudy Morrison, Ph.D., professor of Microbiology & Physiological Systems, said her work on a VLP-based respiratory syncytial virus vaccine that can be modified to COVID-19 causes severe lower respiratory tract disease in young children and the elderly. And some of the problems inherent in the production of vaccines from inactivated or live viruses will be avoided [ 97 ].
Medicago, a biopharmaceutical company, headquartered in Quebec City, announced the successful development of a coronavirus VLP only 20 days after the SARS-CoV-2 (COVID-19 disease virus) gene was obtained [ 29 ]. The manufacturing of VLP is the first step in the development of the COVID-19 vaccine, which will now undergo preclinical protection and efficacy testing. They plan to negotiate clinical testing of the vaccine with the relevant health authorities by summer (July/August) 2020 once this is done. Medicago uses its technology platform to create antibodies against SARS-CoV-2. These antibodies to SARS-CoV-2 might theoretically be used to treat people who are infected by the virus. In part, this study is sponsored by the Canadian Institutes for Health Research [ 98 ]. In Table 5 , potential VLPs-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 81 , 99 ].
Table 5. Potential VLPs-based vaccine candidates for COVID-19 in the clinical development pipeline.
VLP, virus-like particle; COVID-19, coronavirus disease 2019; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HBsAg, Hepatitis B surface antigen.
Current Updates
To bring this pandemic to an end, a large share of the world needs to be immune to the virus. The safest way to achieve this is with a vaccine. Vaccines are a technology that humanity has often relied on in the past to bring down the death toll of infectious diseases. Within less than 12 months after the beginning of the COVID-19 pandemic, several research teams rose to the challenge and developed vaccines that protect from SARS-CoV-2, the virus that causes COVID-19. Now the challenge is to make these vaccines available to people around the world.
To resume a normal lifestyle, free from government lockdowns, and fear of continuing pandemic waves over the coming months, the world is anxiously awaiting a safe, successful vaccine to protect against COVID-19. Innovative ties with both pharmaceutical companies and medical start-ups are joining hands with scientists across the continents to repurpose medications, create vaccines, and devices to hinder the progress of this overwhelming pandemic. A large number of vaccine candidates for COVID-19 based on different platforms have already been identified. Current review shows preclinical as well as in clinical development of vaccine candidates, wherein, five major vaccine platforms for COVID-19 namely RNA, DNA, viral vector, protein subunit, and VLP which constitutes 10, 2, 10, 14, and 2 vaccine candidates globally in clinical development as of 15 October 2020. Among all the vaccine platforms, extensive research and development are going on protein subunit-based vaccine which has the maximum candidates in the clinical development.
A significant amount of hindrance to the rapid production of vaccines is the length of clinical trials. With several phases, including the preclinical stage and clinical development, which is a three-phase process, the vaccine development process is very laborious. However, if adequate data is already available, it has been proposed that a few stages be skipped to accelerate the achievement of a vaccine faster with a rapid regulatory review, approval, development, and quality control. By looking towards pandemic conditions, the scientific fraternity will be ready for any harmful situation to overwhelmed opportunities. Therefore, the current situation has given the world a new perspective to facilitate research in the worst circumstances and hasten the drug development process.
No potential conflict of interest relevant to this article was reported.
- 1. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Dhama K, Sharun K, Tiwari R, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16:1232–1238. doi: 10.1080/21645515.2020.1735227. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. World Health Organization. The access to COVID-19 tools (ACT) accelerator [Internet] Geneva: World Health Organization; 2020. [cited 2020 Oct 23]. Available from: https://www.who.int/initiatives/act-accelerator . [ Google Scholar ]
- 8. World Health Organization. COVID-19 vaccines: the push for a COVID-19 vaccine [Internet] Geneva: World Health Organization; 2020. [cited 2020 Oct 23]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines . [ Google Scholar ]
- 9. Funk CD, Laferriere C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Andre FE. The future of vaccines, immunisation concepts and practice. Vaccine. 2001;19:2206–2209. doi: 10.1016/s0264-410x(00)00546-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Pronker ES, Weenen TC, Commandeur H, Claassen EH, Osterhaus AD. Risk in vaccine research and development quantified. PLoS One. 2013;8:e57755. doi: 10.1371/journal.pone.0057755. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Sharpe HR, Gilbride C, Allen E, et al. The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology. 2020;160:223–232. doi: 10.1111/imm.13222. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Heaton PM. The Covid-19 vaccine-development multiverse. N Engl J Med. 2020;383:1986–1988. doi: 10.1056/NEJMe2025111. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Van Riel D, de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Craven J. COVID-19 vaccine tracker [Internet] Rockville (MD): Regulatory Affairs Professionals Society; c2021. [cited 2020 Oct 23]. Available from: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker . [ Google Scholar ]
- 21. Begum J, Mir NA, Dev K, Buyamayum B, Wani MY, Raza M. Challenges and prospects of COVID-19 vaccine development based on the progress made in SARS and MERS vaccine development. Transbound Emerg Dis. 2021;68:1111–1124. doi: 10.1111/tbed.13804. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Shang W, Yang Y, Rao Y, Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020;5:18. doi: 10.1038/s41541-020-0170-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): prevention & treatment. Atlanta (GA): Centers for Disease Control and Prevention; 2019. [ Google Scholar ]
- 25. Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. doi: 10.3390/jcm9030623. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Dhama K, Patel SK, Pathak M, et al. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis. 2020;37:101755. doi: 10.1016/j.tmaid.2020.101755. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines: a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Jahanafrooz Z, Baradaran B, Mosafer J, et al. Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today. 2020;25:552–560. doi: 10.1016/j.drudis.2019.12.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report [Internet] New Haven (CT): MedRxiv; 2020. [cited 2020 Oct 23]. Available from: [ DOI ] [ Google Scholar ]
- 32. Saif LJ. Vaccines for COVID-19: perspectives, prospects, and challenges based on candidate SARS, MERS, and animal coronavirus vaccines. Euro Med J. 2020 May 13; doi: 10.33590/emj/200324. [Epub] [ DOI ] [ Google Scholar ]
- 33. Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30:4414–4418. doi: 10.1016/j.vaccine.2012.04.060. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Tu YF, Chien CS, Yarmishyn AA, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21:2657. doi: 10.3390/ijms21072657. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Hussey C. Moderna ships mRNA vaccine against novel coronavirus (mRNA-1273) for phase 1 study. Cambridge (MA): Moderna; 2020. [ Google Scholar ]
- 36. ClinicalTrials.gov. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT04283461?term=mRNA&cond=COVID-19&draw=2&rank=1%0A . [ Google Scholar ]
- 37. ClinicalTrials.gov. Dose-confirmation study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1273 COVID-19 vaccine in adults aged 18 years and older [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT04405076?term=moderna&cond=covid-19&draw=2 . [ Google Scholar ]
- 38. ClinicalTrials.gov. A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT04470427?term=vaccine&cond=covid-19&draw=5 . [ Google Scholar ]
- 39. A phase I clinical trial of novel coronavirus pneumonia (COVID-19) mRNA vaccine (BNT162b1) in China. [place unknown]: Chinese Clinical Trial Register (ChiCTR); 2018. [ Google Scholar ]
- 40. ClinicalTrials.gov. A trial investigating the safety and effects of one BNT162 vaccine against COVID-19 in healthy adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT04537949?term=vaccine&cond=covid-19&draw=4 . [ Google Scholar ]
- 41. ClinicalTrails.gov. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04368728 . [ Google Scholar ]
- 42. ClinicalTrials.gov. A study to evaluate the safety, reactogenicity and immunogenicity of vaccine CVnCoV in healthy adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04449276?term=vaccine&cond=covid-19&draw=6#contacts . [ Google Scholar ]
- 43. ClinicalTrials.gov. Ascending dose study of investigational SARS-CoV-2 vaccine ARCT-021 in healthy adult subjects [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04480957?term=vaccine&cond=covid-19&draw=10 . [ Google Scholar ]
- 44. Imperial College London. Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: [ DOI ] [ Google Scholar ]
- 45. A phase I clinical trial to evaluate the safety, tolerance, and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18–59 years and 60 years and above. [place unknown]: Chinese Clinical Trial Register (ChiCTR); 2018. [ Google Scholar ]
- 46. Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Iqbal Yatoo M, Hamid Z, Parray OR, et al. COVID-19: recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccin Immunother. 2020;16:2891–2904. doi: 10.1080/21645515.2020.1788310. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Van Doremalen N, Haddock E, Feldmann F, et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 2020;6:eaba8399. doi: 10.1126/sciadv.aba8399. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. UW-Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine [Internet] San Francisco (CA): Business Wire; 2020. [cited 2020 Oct 24]. Available from: https://www.businesswire.com/news/home/2020040-2005666/en/UW-Madison-FluGen-Bharat-Biotech-to-develop-CoroFlu-a-coronavirus-vaccine . [ Google Scholar ]
- 53. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. ClinicalTrials.gov. A study to evaluate the safety and immunogenicity of COVID-19 (AdimrSC-2f) vaccine [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04522089 . [ Google Scholar ]
- 55. Guimaraes CA. Clinical trials register. Rev Col Bras Cir. 2007;34:201–204. [ Google Scholar ]
- 56. ISRCTN Registry. A phase III study to investigate a vaccine against COVID-19: ISRCTN89951424 [Internet] [place unknown]: ISRCTN Registry; 2020. [cited 2020 Oct 24]. Available from: http://www.isrctn.com/ISRCTN89951424 . [ Google Scholar ]
- 57. ClinicalTrials.gov. Phase III double-blind, placebo-controlled study of AZD1222 for the prevention of COVID-19 in adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04516746 . [ Google Scholar ]
- 58. ClinicalTrials.gov. GRAd-COV2 vaccine against COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04528641 . [ Google Scholar ]
- 59. ClinicalTrials.gov. A clinical trial of a recombinant adenovirus 5 vectored COVID-19 vaccine (Ad5-nCoV) with two doses in healthy adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04552366?term=vaccine&cond=covid-19&draw=3 . [ Google Scholar ]
- 60. ClinicalTrials.gov. Trial for safety and immunogenicity of a chikungunya vaccine, VRC-CHKVLP059-00-VP, in healthy adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02562482?term=chikungunya&phase=0124&rank=4 . [ Google Scholar ]
- 61. ClinicalTrials.gov. Evaluating the safety, tolerability and immunogenicity of bacTRL-spike vaccine for prevention of COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04334980 . [ Google Scholar ]
- 62. Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel) 2020;8:153. doi: 10.3390/vaccines8020153. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Cao Y, Zhu X, Hossen MN, Kakar P, Zhao Y, Chen X. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat Commun. 2018;9:3695. doi: 10.1038/s41467-018-06151-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Oscherwitz J. The promise and challenge of epitope-focused vaccines. Hum Vaccin Immunother. 2016;12:2113–2116. doi: 10.1080/21645515.2016.1160977. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Novavax to commence human trial for Covid-19 vaccine [Internet] London: Clinical Trials Arena; 2020. [cited 2020 Oct 24]. Available from: https://www.clinicaltrialsarena.com/news/novavax-covid-19-vaccine-trial/ [ Google Scholar ]
- 69. Lee J. These 23 companies are working on coronavirus treatments or vaccines: here's where things stand [Internet] New York (NY): MarketWatch; 2020. [cited 2020 Oct 24]. Available from: https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06 . [ Google Scholar ]
- 70. Kim E, Erdos G, Huang S, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Arora K, Rastogi R, Arora NM, et al. Multi-antigenic virus-like particle of SARS CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate [Internet] Laurel Hollow (NY): bioRxiv; 2020. [cited 2020 Oct 24]. Available from: [ DOI ] [ Google Scholar ]
- 72. ClinicalTrials.gov. Evaluation of the safety and immunogenicity of a SARS-CoV-2 rS (COVID-19) nanoparticle vaccine with/without Matrix-M adjuvant [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04368988#contacts . [ Google Scholar ]
- 73. ClinicalTrials.gov. A study looking at the effectiveness and safety of a COVID-19 vaccine in South African adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04533399?term=vaccine&cond=covid-19&draw=7 . [ Google Scholar ]
- 74. Phase I clinical study of recombinant novel coronavirus vaccine (CHO cells): NCT04445194 [Internet] London: Cochrane Library; 2020. [cited 2020 Oct 24]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02129626/full . [ Google Scholar ]
- 75. ClinicalTrials.gov. Recombinant new coronavirus vaccine (CHO cells) to prevent SARS-CoV-2 phase I clinical trial (≥60 years old) [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04550351?term=Anhui+Zhifei+Longcom&draw=2&rank=2 . [ Google Scholar ]
- 76. ClinicalTrials.gov. KBP-201 COVID-19 vaccine trial in healthy volunteers [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04473690?term=vaccine&cond=covid-19&draw=3 . [ Google Scholar ]
- 77. ClinicalTrials.gov. Study of recombinant protein vaccine formulations against COVID-19 in healthy adults 18 years of age and older [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04537208?term=sanofi+gsk&draw=4&rank=29 . [ Google Scholar ]
- 78. ClinicalTrials.gov. A study to evaluate the safety and immunogenicity of MVC-COV1901 against COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04487210?term=vaccine&cond=covid-19&draw=7 . [ Google Scholar ]
- 79. ClinicalTrials.gov. SCB-2019 as COVID-19 vaccine [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04405908#contacts . [ Google Scholar ]
- 80. ClinicalTrials.gov. Monovalent recombinant COVID19 Vaccine [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04453852?term=vaccine&cond=covid-19&draw=5 . [ Google Scholar ]
- 81. Taylor N. Trial registered on ANZCTR [Internet] Camperdown: Australian New Zealand Clinical Trials Registry; 2019. [cited 2020 Oct 24]. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375587%0A . [ Google Scholar ]
- 82. ClinicalTrials.gov. Study of the safety, reactogenicity and immunogenicity of “EpiVacCorona” vaccine for the prevention of COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT-04527575?view=record . [ Google Scholar ]
- 83. ClinicalTrials.gov. Study of the safety, reactogenicity and immunogenicity of “EpiVacCorona” vaccine for the prevention of COVID-19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT-04527575?view=record . [ Google Scholar ]
- 84. Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel) 2019;7:37. doi: 10.3390/vaccines7020037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Hobernik D, Bros M. DNA vaccines: how far from clinical use? Int J Mol Sci. 2018;19:3605. doi: 10.3390/ijms19113605. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. ClinicalTrials.gov. Safety, tolerability and immunogenicity of INO-4800 followed by electroporation in healthy volunteers for COVID19 [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04447781#contacts . [ Google Scholar ]
- 90. ClinicalTrials.gov. Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04336410 . [ Google Scholar ]
- 91. ClinicalTrials.gov. Study of COVID-19 DNA vaccine (AG0301-COVID19) [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04463472 . [ Google Scholar ]
- 92. ClinicalTrials.gov. Study of COVID-19 DNA vaccine (AG-0302-COVID19) [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04527081?term=vaccine&cond=covid-19&draw=7 . [ Google Scholar ]
- 93. Clinical Trials Registry India. Novel corona virus-2019-nCov vaccine by intradermal route in healthy subjects [Internet] [place unknown]: Clinical Trials Registry India; 2020. [cited 2020 Oct 24]. Available from: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45306 . [ Google Scholar ]
- 94. ClinicalTrials.gov. Safety and immunogenicity study of GX-19, a COVID-19 preventive DNA vaccine in healthy adults [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04445389?term=vaccine&cond=covid-19&draw=3 . [ Google Scholar ]
- 95. Donaldson B, Lateef Z, Walker GF, Young SL, Ward VK. Virus-like particle vaccines: immunology and formulation for clinical translation. Expert Rev Vaccines. 2018;17:833–849. doi: 10.1080/14760584.2018.1516552. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Xu R, Shi M, Li J, Song P, Li N. Construction of SARS-CoV-2 virus-like particles by mammalian expression system. Front Bioeng Biotechnol. 2020;8:862. doi: 10.3389/fbioe.2020.00862. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Spencer SE. Virus-like particles developed for RSV may hold key to COVID-19 vaccine, UMMS researcher says [Internet] Worcester (MA): UMass Medical School; 2020. [cited 2020 Oct 24]. Available from: https://www.umassmed.edu/news/news-archives/2020/05/virus-like-particles-developed-for-rsv-may-hold-key-to-covid-19-vaccine-umms-researcher-says/ [ Google Scholar ]
- 98. Medicago Inc. MEDICAGO announces production of a viable vaccine candidate for COVID-19 [Internet] Quebec City: Medicago Inc.; 2020. [cited 2020 Oct 24]. Available from: https://www.medicago.com/en/media-room/medicago-announces-production-of-a-viable-vaccine-candidate-for-covid-19/ [ Google Scholar ]
- 99. ClinicalTrials.gov. Safety, tolerability and immunogenicinity of a coronavirus-like particle COVID-19 vaccine in adults aged 18-55 years [Internet] Bethesda (MD): National Library of Medicine; 2020. [cited 2020 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04450004?term=vaccine&cond=covid+19&draw=2#contacts . [ Google Scholar ]
- View on publisher site
- PDF (362.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
Discussion. A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with ...
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and ...
Results. Thirteen randomized, blinded, controlled trials, which involved the safety and efficacy of 11 COVID-19 vaccines, were included. In 10 studies, the 28-day seroconversion rate of subjects exceeded 80%. In two 10 000-scale clinical trials, the vaccines were effective in 95% and 70.4% of the subjects, respectively.
Gam-COVID-Vax Sputnik V: Gamaleya Research Institute: ... TF wrote the first draft of the paper. All authors (TF, YK, CJM, JG, NPS) contributed to the data interpretation, revised each draft for important intellectual content, and read and approved the final manuscript. ... COV2.S COVID Vaccine medRxiv. 2021 09.10.21263385. [Google Scholar] 62.
Our understanding of COVID-19 vaccinations and their impact on health and mortality has evolved substantially since the first vaccine rollouts. Published reports from the original randomized phase 3 trials concluded that the COVID-19 mRNA vaccines could greatly reduce COVID-19 symptoms. ... 4 Immunology and Public Health Research, Independent ...
Since the outbreak of the Coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus in late 2019, substantial research has been undertaken to uncover the health consequences ...
Objectives This meta-analysis evaluated the Efficacy and Effectiveness of several COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson, to better estimate their immunogenicity, benefits, or side effects. Methods Studies reporting the Efficacy and Effectiveness of COVID-19 vaccines from November 2020 to April 2022 were included. The pooled Effectiveness ...
We matched vaccine recipients and controls on variables associated with the probability of both vaccination and infection or severity of Covid-19: age, sex, sector (general Jewish, Arab, or ultra ...
COVID-19 vaccine efficacy or effectiveness against severe disease remained high, although it did decrease somewhat by 6 months after full vaccination. By contrast, vaccine efficacy or effectiveness against infection and symptomatic disease decreased approximately 20-30 percentage points by 6 months. The decrease in vaccine efficacy or effectiveness is likely caused by, at least in part ...
The first COVID-19 vaccine was delivered outside of a clinical trial setting on Dec 8, 2020. 1 By Dec 8, 2021, 55·9% of the global population was estimated to have received at least one dose of a COVID-19 vaccine, 45·5% estimated to have received two doses, and 4·3% estimated to have received a booster dose. 2 Despite the incredible speed ...
Pfizer's COVID-19 vaccine was the quickest vaccine to be developed, taking just about 7 months after its phase I/II trial took place in May 2020 for the FDA to allow for its emergency use in December 2020. 8 The previous record set by pharmaceutical company Merck took 4 years to develop the world's first effective vaccine against mumps in ...
Participants were enrolled from December 28, 2020 (2 weeks after the introduction of a Covid-19 vaccine), through May 19, 2021, at 33 sites across 25 U.S. states, representing more than 500,000 ...
6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected]. ... Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and ...
The current COVID-19 pandemic has urged the scientific community internationally to find answers in terms of therapeutics and vaccines to control SARS-CoV-2. Published investigations mostly on SARS-CoV and to some extent on MERS has taught lessons on vaccination strategies to this novel coronavirus. This is attributed to the fact that SARS-CoV ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
594/2021 Manuel Bagues and Velichka Dimitrova, We quantify the impact of COVID-19 vaccination on psychological well-being using information from a large-scale panel survey representative of the UK population. Exploiting exogenous variation in the timing of vaccinations, we find that vaccination increases psychological well-being by 0.12 standard deviation, compensating for around one half of ...
Background. Parent vaccine hesitancy is a sensitive topic despite the benefits associated with children's vaccination. Especially regarding the COVID-19 vaccination, parents displayed concerns about children's vaccination, questioning their effectiveness and security. Although several studies were conducted on the general population, few studies investigated this relationship on parents ...
A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19. The study was designed to primarily evaluate the efficacy, safety, and immunogenicity of mRNA-1273 to prevent COVID-19 for up to 2 years after the second dose of mRNA-1273.