

Conservation of Mass Experiments

Start with reviewing the difference between physical and chemical changes. (Chemical changes include: gas, color change, precipitate, temperature change, or light). Get some play doh and roll it into a ball. Place it on the scale and ask students if they think the mass will change if you change the shape of the play doh. You could also use legos or anything else you have handy.
Once they’ve seen that physical changes don’t cause a mass change, move on to chemical changes. Here are some labs you can use for different grade levels to teach the law of conservation of mass.

- Read more about: Chemistry , Experiments

Hi, I'm Becca!
Search the site, browse by category.
- A list of ALL blog posts
- Back to School
- Biochemistry
- Body Systems
- Classification
- Classroom Decor
- Classroom Management
- Distance Learning
- End of the School Year
- Experiments
- Field Trips
- For NEW Teachers
- Formative Assessment
- Media in the Classroom
- Microscopes
- Photosynthesis & Respiration
- Plate Tectonics
- Sustainability
- Teacher Tips
- Weather and Climate
Get Freebies!
You might also like....

Science Seek and Finds

Layers of the Earth Lessons

Teaching the Electromagnetic Spectrum

Want a fun way to practice science vocabulary? Try out seek and finds!

Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
How does burning magnesium affect its mass? | 11-14 years
- No comments
Investigate what happens to the mass of magnesium when it burns and reacts with oxygen using this lesson plan and practical activity for 11–14 year olds
Students explore, in a practical activity, what happens to the mass of magnesium when it burns. This helps them consolidate their ideas about the increase in mass on burning due to combination with oxygen. Students also evaluate and develop their ability to draw and interpret simple graphs.
Learning objectives
Students will be able to:
- Describe and explain what happens to the mass of magnesium when it burns.
- Draw and interpret simple graphs.
Sequence of activities
Demonstration and introduction.
- Issue a blue safety glass to each student.
- Ignite a piece of magnesium ribbon over a cement mat. Ensure that students use the safety glass to observe this.
- Increase – green
- Stay the same – yellow
- Decrease – red
- A student to explain their answer.
- Other students to add to this if appropriate.
- The learning objectives.
- That they are going to investigate what happens to the mass of magnesium when it is burnt.
Practical: set-up
Describe the practical details, with students to work in pairs and follow safe procedures to:
- Weigh a crucible and lid.
- Add a prepared piece of magnesium ribbon to the crucible and weigh it again.
- Place the crucible on a pipeclay triangle supported on a tripod and heat it with a Bunsen burner flame, lifting the lid of the crucible from time to time using a pair of tongs.
- Let the crucible cool and weigh it again.
- Place the crucible on the pipeclay triangle again and re-heat for a short time, let it cool and reweigh.
Circulate and support while pairs:
- Carry out the practical.
- Add their results to a class set written on a board or projected.
- Write them down on their copy of the ’Results table’ sheet.
- Complete all parts of their ’Results table’.
A set of specimen results is provided that may be used if student data is not available (see downloads below).
Graphs exercise
Discuss with the whole class What makes a good graph? Refer to the criteria on their ‘Results table’ sheet.
Support individuals as they:
- Draw the graph of mass of magnesium against mass of magnesium oxide formed.
- Check that their graph meets the expected standard, and modify it if necessary.
- Work with the other member of the pair to discuss whether they think that the graph meets the required standard.
- Offer help to their partner only in the form of questions.
- Confirm with you that the graph is up to standard.
- Answer a question that you pose, if it is not to standard.
- Use their graphs to predict how much magnesium oxide would be produced from a specified mass of magnesium.
- Write in words on their ’Results table’ sheet how they can use their graph to predict the mass of magnesium oxide which will be formed from a particular mass of magnesium when it is burnt.
Take in the graphs and written sheets and comment on any features that the student still needs to develop.
Sharing objectives and criteria gives purpose to the activity and a framework for self criticism. Through the discussion about their own and their partner’s graphs, students will see how to improve. When interpreting their graph, students will see why certain standards need to be met.
Teacher feedback is used to affirm standards or to prompt improvement.
Practical notes
- Blue safety glass
- Set of traffic light cards
- Graph paper
For the practical (see note 4 in ‘Health, safety and technical notes’ below):
- Crucible and lid
- Pipeclay triangle
- Bunsen burner
- 10–20 cm lengths of magnesium ribbon (FLAMMABLE) ranging from 0.1 g to 0.5 g
- Access to a balance measuring to 0.01 g
Health, safety and technical notes
- Read our standard health and safety guidance .
- Wear eye protection.
- It is the responsibility of the teacher to carry out an appropriate risk assessment.
- The ‘Set of specimen results’ handout can be used in place of carrying out the practical, or if student data is not available (see downloads below).
Principal hazards
- Crucibles remaining hot for some time (leave to cool on the tripods).
- Magnesium ribbon flammability.
Results table
Set of specimen results, specimen results with answers, specimen graph, additional information.
This lesson plan was originally part of the Assessment for Learning website, published in 2008.
Assessment for Learning is an effective way of actively involving students in their learning. Each session plan comes with suggestions about how to organise activities and worksheets that may be used with students.
- 11-14 years
- Practical experiments
- Formative assessment
- Lesson planning
- Practical skills and safety
- Quantitative chemistry and stoichiometry
- Reactions and synthesis
Specification
- 1. Investigate whether mass is unchanged when chemical and physical changes take place.
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- Students should be able to explain any observed changes in mass in non-enclosed systems during a chemical reaction given the balanced symbol equation for the reaction and explain these changes in terms of the particle model.
- The law of conservation of mass states that no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants.
- Recall and use the law of conservation of mass.
- Explain any observed changes in mass in non-enclosed systems during a chemical reaction and explain them using the particle model.
- 1.47b Explain the law of conservation of mass applied to: a non-enclosed system including a reaction in an open flask that takes in or gives out a gas
- C5.3.1 recall and use the law of conservation of mass
- C5.3.2 explain any observed changes in mass in non-enclosed systems during a chemical reaction and explain them using the particle model
- C3.1b use the names and symbols of common elements and compounds and the principle of conservation of mass to write formulae and balanced chemical equations and half equations
- C1.3j explain any observed changes in mass in non-enclosed systems during a chemical reaction and explain them using the particle model
- C5.2.1 recall and use the law of conservation of mass
- C5.2.2 explain any observed changes in mass in non-enclosed systems during a chemical reaction and explain them using the particle model
- C1.3l explain any observed changes in mass in non-enclosed systems during a chemical reaction and explain them using the particle model
- The mass of a mole of any substance, in grams (g), is equal to the gram formula mass and can be calculated using relative atomic masses.
- (p) how to calculate the formula of a compound from reacting mass data
- 2.2.1 recognise oxidation and reduction in terms of loss or gain of oxygen or hydrogen and identify in a reaction or symbol equation which species is oxidised and which is reduced (link to suitable industrial processes covered in this specification);
Related articles

Help learners master equilibrium and reversible reactions
2024-06-24T06:59:00Z By Emma Owens
Use this poster, fact sheet and storyboard activity to ensure your 14–16 students understand dynamic equilibrium

Non-burning paper: investigate the fire triangle and conditions for combustion
2024-06-10T05:00:00Z By Declan Fleming
Use this reworking of the classic non-burning £5 note demonstration to explore combustion with learners aged 11–16 years

Everything you need to introduce alkenes
2024-06-04T08:22:00Z By Dan Beech
Help your 14–16 learners to master the fundamentals of the reactions of alkenes with these ideas and activities
No comments yet
Only registered users can comment on this article., more lesson plans.

Determining the structure of compounds | 16–18 years
Examine data relating to the structure and complexity of compounds, including mass, infrared and 1 H NMR spectra

How do scientists grow protein crystals? | 14-16 years
Discover the methods and conditions used by chemical scientists to grow protein crystals in this lesson plan with activities for 14–16 year olds.

How does sodium react with chlorine? | 14-16 years
Investigate the reaction of sodium with chlorine, using students’ understanding of atoms, ions and lattice structure, in this lesson plan for 14–16 year olds.
- Contributors
- Email alerts
Site powered by Webvision Cloud
Experiment 4: The Law of Conservation of Mass
Related documents.

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
Enhancing solar still performance with hybrid nanofluid: a comprehensive assessment of energy, exergy, economics, and environmental impact using a novel fractional model
- Open access
- Published: 24 August 2024
Cite this article
You have full access to this open access article

- E. F. El-Gazar 1 ,
- Mohamed S. Yousef 2 ,
- Abdelrahman M. Elshaer 3 ,
- Mahmoud A. Khattab 1 ,
- T. A. Mouneer 3 &
- A. A. Hawwash 3
In the present study, the thermal performance of a modified solar still (MSS) system coupled with hybrid nanofluid (HNF) of titanium oxide (TiO 2 ) and silicon oxide (SiO 2 ) has been investigated theoretically based on energetic, exergetic, economic, and enviroeconomic assessment. The model of the MSS has been introduced using a new numerical technique of the Atangana-Baleanu fractional derivative. The fractional model of the MSS system is presented under various weather circumstances (winter and summer seasons) in Egypt to show the impact of HNF on the MSS output: temperatures, freshwater productivity, exergy, and energy efficiencies. The outcomes of the fractional model are contrasted to those derived from actual experimental data collected under varying climatic conditions in Upper Egypt. Numerical findings demonstrate specific consistency between the experimental results and the proposed model of the solar still (SS), with a percentage of error of 4.65% in freshwater productivity. Moreover, using hybrid nano enhances daily productivity in the summertime by 27.2% and in the wintertime by 21.7%, increasing efficiencies. Additionally, a comparative economic and environmental assessment has been investigated for all the proposed desalination systems without and with HNF. The findings found that the cost per liter of MSS was 44% lower than that of the conventional solar still (CSS) during the summer season. Using exergy and energy approaches, MSS reduced CO 2 by 22% and 29.6% more during the winter.
Explore related subjects
- Environmental Chemistry
Avoid common mistakes on your manuscript.
1 Introduction and literature review
In recent years, access to clean, healthy, and pure water is indeed one of the most critical issues facing humanity. Without adequate access to safe water, communities face numerous challenges related to health, sanitation, food security, and economic development.
Approximately 97% of Earth's water is saline, found in oceans and seas, while only about 3% is freshwater. This freshwater exists in various forms, including surface water such as rivers, lakes, and freshwater wetlands, as well as groundwater and frozen water in polar ice caps, glaciers, and snowfields (Abujazar et al., 2016 ; Bait & Si-Ameur, 2018 ; Rashidi et al., 2016 ).
By 2035, over a quarter of the people on our planet will be affected by water scarcity due to the growth in the world's population and the increasing query for water consumption. As a result, desalination technology is regarded as one of the most significant processes for obtaining potable freshwater depending on exploiting the sources of brackish water (seas and oceans). However, most of the desalination technologies that have been developed recently have complex structures and designs. Additionally, most of these desalination systems rely on fossil fuel sources, meaning their operating and maintenance costs will continue to rise dramatically due to the limited resources for fossil fuels. Energy storage materials could used as a source of heat inside the SS during the night (Hawwash et al., 2023 ; Hawwash et al., Sep. 2019 ). The SS is a simple and economical design for producing clean water, which depends on the incident solar radiation to convert salty impure water into filtered water (El-Gazar et al., 2020 ).
Hussen et al. ( 2023 ) investigated four distinct designs of the SSs. The tested SS systems were conventional, pyramid, double slope, and tubular SS. For mitigating the effect of shadow inside the double slope SS and pyramid SS leading to a rise inside the water basin. In addition, Phase Change Materials (PCM) were employed to enhance thermal storage performance. The design of pyramid SS with PCM showed the greatest values of 116.4%/51.3%, for productivity and thermal efficiency, respectively. Aly et al. ( 2023 ) enhanced the output of tabular SS by presenting a new design, the oval tubular SS coupled with PCM and cover plate cooling. The productivity and efficiency of the oval tubular SS were enhanced by 32.42% and 41.26%, respectively.
Alshqirate et al. ( 2023 ) improved the freshwater productivity of conventional SS with natural Palmately leaf fibers. The outcomes found that the daily total freshwater yield was 5160.8 gm/m 2 by using natural fiber, with an increasing percentage of 44.5% relative to the CSS. Manoj Kumar et al. ( 2022 ) conducted an experiment investigating the effect of thermal energy storage/ SS integration. Inorganic PCM and nano-doped PCM were integrated with a single-sloped conventional SS. The findings showed that using inorganic PCM and nano/PCM composite boosted the percentage of freshwater yield from the SS by 26.63% and 45.23%, respectively. Hameed et al. ( 2022 ) evaluated the thermal performance of single slope SS integrated with fins of a square area involved in the absorber plate. The work also studied the efficacy of SS glass cover cooling methodology. The findings reported an improvement in the water productivity of the SS/fins combination by 40%. Also, the study reported a significant improvement in productivity with cover cooling.
Ahmed et al. ( 2022 ) investigated the influence of cotton wick material integrating with tubular SS and parabolic concentrator systems. The results revealed that the cotton wick enhanced the setup efficiency and productivity by 24.45% and 29.11%, respectively. Angappan et al. ( 2023 ) studied the integration of SS and box-shaped solar cookers to boost SS water productivity. The outcomes reported that the conventional and modified SS daily productivities reached nearly 4 L/m 2 and 6 L/m 2 , respectively, with an increasing percentage of 41% compared to the conventional one. Chauhan et al. ( 2023 ) improved the SS output by surface coating of Lauric acid and phosphorus quantum dot material. Two designs of SSs were adopted, the conventional SS and prism-shaped SS. The results showed that traditional and prism-shaped SS with PCM and black coating yielded about 3050 ml/m 2 and 3600 ml/m 2 , respectively.
Abdullah et al. ( 2023 ) modified a single slope SS by integrating Nano- PCM, with an external condenser, copper water heating coil, internal, and external reflectors to enhance freshwater productivity. Combining external reflectors (top and bottom) with external condenser, and PCM improved the SS productivity by about 42%, 57%, and 41%, respectively. Kumaravel et al. ( 2022 ) investigated the performance analysis of a single slope SS integrated with thermal energy storage with blue metal stones and pebble stones. The findings showed that combining the two types of rocks and their integration with the SS increased productivity by 18% relative to conventional SS.
Much research was dedicated to enhancing SS's thermal and productivity performance by adopting numerical and mathematical models. Moustafa et al. ( 2022 ) increased the tubular SS's water productivity and energy efficiency by attaching an electric heater to the still basin powered by a solar photovoltaic (PV) panel (El-Gazar et al., 2023a ). Also, the work developed a model based on artificial intelligence (AI) for predicting the SS's water yield and thermal efficiency. The findings revealed that the modified SS had an average daily accumulated water productivity of nearly 3 L/m 2 with a 31.85% enhancement percentage. Mittal ( 2021 ) delivered a Computational Fluid Dynamics (CFD) modeling of a single slope SS using ANSY S Fluent. The CFD model approach computationally proved its effectiveness in capturing the distillate water output at various configurations, provided that the bottom and top surface temperatures are identified. Mohsenzadeh et al. ( 2022 ) established and validated a transient model experimentally to investigate a passive SS. The evaporation chamber was designed with different aspect ratios, and all the cases were investigated. The study revealed that the aspect ratio of the SS evaporation chamber increased and produced more fresh water.
Lisboa et al. ( 2022 ) performed a model analysis for the freshwater productivity enhancement techniques of SSs. The study concluded that the dominant factors that enhance water productivity were structure design, water depth, basin-glass temperature difference, insulation, and solar irradiation. Keshtkar et al. ( 2020 ) introduced a CFD mathematical model to study the impact of different design factors of passive basin SS. The findings show that the SS freshwater yield increased by about 14% when the wind velocity was raised by 5 m/s. The SS productivity also increased by 3.5% when the glass width decreased to 2 mm. Hassan et al. ( 2022 ) provided a novel simulation model for estimating SS's performance. The model results were proven experimentally, and different materials of SS walls were studied. The water productivity per unit area of the basin was about 2 and 4 kg/m 2 for glass and wooden walls, respectively. A numerical simulation was modeled by Gupta et al. ( 2022 ) to investigate the performance of a single slope SS under different climate conditions in India. The model accurately predicted the SS's performance at distinct conditions.
Nanofluids were used to increase the SS efficiency in more studies (Ajit et al., 2023 ; Chen et al., 2023 ; Mustafa et al., 2023 ; Sahu & Tiwari, 2024 ). Kabeel et al. ( 2019 ) investigated experimentally a new absorber plate of a pyramid SS covered with titanium oxide nanoparticles black coating. The findings summarized that water yield was increased by the decrease in the water depth. The coated absorber plate improved the freshwater yield by 12% at maximum water depth. Rashidi et al. ( 2018 ) examined numerically the influence of nanofluid insertion on the freshwater output of a stepped SS. The outcomes showed that raising the nanoparticle percentage by 5% increased the hourly water productivity by about 22%. Sahota et al. ( 2019 ) investigated the performance of a double slope SS with alumina and Multi-Wall Carbon Nano Tubes (MWCNTs) water-based nanofluid. The results found a remarkable improvement in SS's evaporative heat transfer coefficient and freshwater output by adopting water-based nanofluids.
Sharshir et al. ( 2022 ) conducted an experimental study investigating the performance of pyramid SS integrated with evacuated tubes, nanofluid, external condensers, and ultrasonic foggers. The results showed that modified SS with six evacuated tubes, nanofluid, and an external condenser had increased the freshwater yield, exergy efficiency, and energy efficiency by about 132%, 75%, and 28%, respectively. Alsehli et al. ( 2022 ) implemented a new design for SS and improved freshwater productivity by incorporating graphene nanoplatelet/platinum hybrid nanofluid. The study found that mixing the nanoparticle with the base fluid led to a remarkable growth in freshwater productivity.
Mustafa et al. ( 2023 ) performed a two-phase analysis on integrating aluminium-based nanofluid in SS. AAluminumnanoparticles were utilized in the SS's basin. The results concluded that solar irradiation dominated the PCM maximum temperature, air temperature, and PCM volume fraction. Maatki et al. ( 2022 ) enhanced the heat transfer within a triangular SS using Carbon Nano Tube (CNTs)—based nanofluid. The results showed that the MSS design with nanofluid significantly improved the mass and heat transfer rates. Modi et al. ( 2022 ) examined the effect of thermal energy storage materials and nanoparticles on the freshwater productivity of pyramid SS with square shape. The outcome revealed that the SS's total water productivity and efficiency with energy storage were greater by 5% and 5.5%, respectively than nanofluid.
The research adopted hybrid nano-based water for enhancing SS productivity is outlined in the following paragraphs. A fractional modeling technique was adopted by El-Gazar et al. ( 2021b ) to examine the impact of HNF on the performance of a conventional SS. The HNFs adopted were alumina and copper oxide. The results revealed that adopting hybrid nanofluids increased the freshwater's daily productivity by nearly 27% in hot weather conditions and 21% in cold conditions. Shoeibi et al. ( 2022 ) numerically evaluated the cover glass cooling of a double-slope SS integrated with HNF (TiO 2- Al 2 O 3 ). The results revealed that the productivity of freshwater improved by 11.09%, compared to the model without a hybrid nanofluid. Rabbi et al. ( 2021 ) improved the SS's performance using two-hybrid nanofluids. The freshwater productivity, and efficiency, were 4.99 kg m −2 / day, 37.76%, respectively when water was mixed with Al 2 O 3 –SiO 2 hybrid nanofluid. El-Gazar et al. ( 2021b ) conducted a novel nonlocal model for SS performance investigation integrated with a PV panel, HNF, and saline water preheating. The findings show that the daily SS's productivity was 7.1 kg/m 2 per day when the HNF was employed, leading to an improvement percentage of 10% relative to the SS system without a hybrid nanofluid. Kaviti et al. ( 2023 ) studied the water desalination system of a SS using HNF of cerium oxide (CeO 2 ) nanoparticles and MWCNTs. The modified SS accomplished a high productivity of 1430 mL relative to the CSS, with a peak productivity of 920 mL.
The study of the SS systems did not stop with enhancing the yield and efficiency of the still. Still, it also included work on exergetic analysis, which mainly depends on thermodynamics's second law. Compared to energetic analysis, which relies on the 1st law of thermodynamics, exergy assessment appears to be an effective observation tool for investigating the optimization, performance, thermal design, and evaluation of various energy techniques (Asbik et al., 2016 ; Gad et al., 2022 ; Hakim et al., 2018 ; Ibrahim et al., 2017 ; Jafarkazemi & Ahmadifard, 2013 ; Kianifar et al., 2012 ; Kumar et al., 2020 ; Ranjan et al., 2016 ). Indeed, exergy analysis offers valuable insights into the performance of solar still (SS) systems by identifying locations, magnitudes, and types of irreversibilities and losses within the system. Unlike energy analysis, which focuses solely on quantity, exergy analysis considers the quality of energy and provides a more comprehensive understanding of system efficiency. Pal et al. ( 2018 ) presented a thermal model for the double slope SS, then studied the exergy and energy assessment for the still system after adding black cotton wicks in the still basin to enhance its efficiency. Another new fractional model for the SS system, which studied the energy and exergy assessment, was presented by El-Gazar et al. ( 2021a ). The fractional model was presented to show the effect of mixing hybrid nanoparticles with salty water on the exergy and energy efficiencies of a SS system coupled with a PV panel. Moreover, Sharshir et al. ( 2018 ) compared the conventional SS and the modified still with nanoparticles of copper oxide (CuO) and graphite. The comparison was presented based on evaluating the energetic, energetic, and economic parameters for both the SS with and without using the nanofluid.
Although it is widely acknowledged that the exergy technique may be utilized to represent the performance of SS systems effectively, there are still certain limitations and weaknesses in environmental and economic analyses (Abo-Elfadl et al., 2021 ; Deniz & Çınar, 2016 ; Gaur & Tiwari, 2014 ; Hassan et al., 2020 ; Joshi & Tiwari, 2018 ; Singh, 2018 ; Singh & Tiwari, 2017 ; Yousef et al., 2022 ). So, the work on the energy systems has been expanded to include how the desalination system affects the environment and how much -effectiveness it costs. Environmental and economic approaches afford in-depth insight into the cost analysis of the engineering processes and the environmental impact by recognizing the necessary tools in the work conditions. Considering the abovementioned, exergy-economic and environmental approaches are being rapidly applied to evaluate different solar distillations. Among these works, which have been developed recently, was conducted by El-bar et al. ( 2019 ), where they studied the (4E) exergy-economic, enviro-economic energy, and exergy, analysis for SS systems integrated with the photovoltaic panel. Based on the environmental approach, the results showed that the conventional still mitigated about 18.99 tons of CO 2 per year, whereas the still coupled PV mitigated 20.89 tons of CO 2 .
Yousef and Hassan ( 2018 ) studied the performance of a solar distilled system incorporated with energy storage material (PCM) from exergy-environmental and exergy-economic points of view. According to the findings of this study, it was found that the SS system with the PCM material was found to be greener than the conventional still (without PCM). Recently, Fathy et al. ( 2020 ) assessed the performance of several configurations of single slope SS with condenser and with forced air cooling based on environmental and economic approaches. Moreover, Lovedeep et al. ( 2017 ) analyzed the eco-economic and enviro-economic effects of a solar distillation machine with two basins and several types of nanofluids. The findings showed that there was an increase in annual freshwater yield by 5.2%, 10.4%, and 12% by using CuO, TiO 2 , and Al 2 O 3 nanofluids, respectively. Additionally, the nanofluid of Al 2 O 3 was reported to have a greater enviro-economic parameter than the other varieties of nanoparticles.
More studies enhanced the effectiveness of SS using nanofluids Ravinder et.al ( 2023 ) investigated the effect of TiO 2 /Jackfruit peel nanofluid with silver balls on the performance of a double-effect solar still. The results presented that the highest efficiency was 20% reached when the energy and exergy were equal 40% and 5.6% respectively, while the depth in water was 0.8 Cm. In another study, a simple stepped solar still was modified three times and investigated experimentally to boost the production rate (Adibi Toosi et al., 2023 ). The PCM, hybrid nano, and magnetic field were added separately to CSS and examined. It is concluded that the production was increased by 98%, 75%, and 37% by the magnetic field, hybrid nano, and PCM respectively, compared with CSS.
Based on the literature and regarding the author's greatest survey, it has been found that although the main importance of the theoretical works is to improve the performance of solar stills and optimize their working condition, very few mathematical models of solar stills have been presented in comparison to the vast number of experimental works. Moreover, it is discovered that the majority of the given numerical models of desalination systems have been calculated based on classical derivatives, which leads to a large error when compared to the actual experimental results. Furthermore, hybrid nanofluids have demonstrated their ability to improve various solar energy systems. The traditional numerical methods may sometimes struggle to accurately model solar still systems, leading to discrepancies between numerical predictions and actual results. So, the high motivation behind the applied method is to introduce a new nonlocal numerical method based on fractional differential equations to accurately describe the thermal performance of the SS systems and show precise outcomes about its output from water productivity and efficiencies with and without a hybrid nanofluid.
2 The problem statement and the objective of the study
The problem statement of the present study is divided into two significant points: Firstly, the limited availability of mathematical models for solar stills compared to experimental works presents a challenge in optimizing their performance. To overcome this limitation, this research introduces a novel nonlocal (fractional) model based on the Atangana Baleanu fractional derivative. Secondly, due to the lower productivity of the solar still system, hybrid nanoparticles of (Silicon oxide SiO 2 / Titanium Oxide TiO 2 ) are used with the saline water inside the still basin to enhance its thermal conductivity, and hence increase its output from the fresh water.
The objective of the current research is summarized in three main points as follows:
Addressing the Gap in Theoretical Modeling: The limited availability of mathematical models for solar stills compared to experimental works presents a challenge in optimizing their performance. To overcome this limitation, this research introduces a novel nonlocal (fractional) model based on the Atangana Baleanu fractional derivative. By embracing fractional calculus principles, this model is expected to provide more precise predictions and bridge the gap between theoretical simulations and actual experimental outcomes. With an accurate representation of the solar still system's thermal behavior, researchers and practitioners can gain deeper insights into system performance and optimize its design and operation more effectively.
Harnessing Hybrid Nanofluids for Enhanced Efficiency: Hybrid nanofluids have shown great promise in enhancing various solar energy systems, making them an attractive avenue for improving solar still performance. By incorporating Silicon Oxide (SiO2) and Titanium Oxide (TiO2) nanoparticles into the briny water, the overall efficiency and productivity of the solar still system can be significantly enhanced. These nanoparticles possess unique heat transfer and energy-absorbing properties, contributing to improved performance metrics. The study meticulously explores the influence of hybrid nanofluids on critical parameters, such as temperatures, freshwater yield, energy efficiency, and exergy efficiency. By thoroughly analyzing the impact of these novel nanofluid-based systems, this research aims to unlock their full potential in real-world applications.
In-Depth Comparative Analysis: The research goes beyond conventional evaluations by conducting a comprehensive comparative analysis encompassing energetic, exergetic, economic, and enviroeconomic parameters. This holistic approach allows for a thorough evaluation of the suggested distilled systems both with and without the implementation of hybrid nanofluids. By considering a wide range of performance metrics, the paper provides a comprehensive assessment of the system's effectiveness, efficiency, economic viability, and environmental impact. This multi-dimensional analysis empowers decision-makers with valuable insights to select the most optimized and sustainable approach for solar desalination.
In the present study, the following points summarized the novelty and contributions of the current work: (i) The suggested fractional (nonlocal) model provides more accurate outcomes and a perfect agreement with the actual data compared to the standard model. (ii) The proportion of inaccuracy between the experimental and theoretical data is reduced by the new non-local model. (iii) hybrid nanoparticles of (Silicon oxide SiO 2 /Titanium Oxide TiO 2 ) have been added to the briny water to enhance the thermophysical characteristics of the conventional fluid and hence raise the solar still's water productivity. (iv) Additionally, the hybrid nanofluid contributes to increasing the traditional SS's exergy and energy efficiencies. (iiv) A comparative study is carried out for all the proposed distillation systems with and without hybrid nanofluid, taking into account energy, exergetic, economic, and enviroeconomic factors.
3 Basic definitions
In this section, various and essential definitions of the fractional operators have been presented as follows (Obembe et al., 2016 ; Sales Teodoro et al., 2019 ; Sun et al., 2019 ; Žecová & Terpák, 2015 ):
Definition 3.1.
The Riemann- Liouville (RL) fractional operator of \(T\left(t\right)\) is (El-Gazar et al., Oct. 2023b ):
where \(\Gamma ( \cdot )\) is the common Gamma function.
Definition 3.2.
The Caputo fractional operator of \(T\left(t\right)\) can be described as.
Definition 3.3.
The Atangana-Baleanu (AB) Caputo type is.
Where \(M\left( \alpha \right)\) considers a function satisfied the relation \(M\left(0\right)=M\left(1\right)=1\) .

4 Fractional model
The energy equilibrium formulas for each of the typical SS's three components, a saline water, absorber plate and glass sheet, result in the thermal model represented in Fig. 1 . The temperature in each region of the solar still serves as the basis for the energy equilibrium equations for that component. The energy equilibrium formulas can be stated by the presumptions given in (Abujazar et al. 2016 ; Bait & Si-Ameur, 2018 ; Rashidi et al., 2016 ).

The Schematic diagram for CSS device
4.1 Glass cover equation
The energy emitted from the glass sheet equals the sum of the energy losses. Hence the glass equation can be described by:
where \({{}_{0}{}^{AB}{\mathbb{D}}}_{t}^{\alpha }\) is the Atangana Baleanu fracrional derivative. The heat transfer coefficients between the atmosphere to the glass are \({h}_{rgl-s}\) and \({h}_{cgl-a}\) , respectively, expressed in W/m 2 .K.
Additionally, the heat transfer coefficients for evaporation, radiation, and convection are \({h}_{cw-gl}\) , \({h}_{rw-gl},\) and \({h}_{ew-gl}\) in W/m 2 .K.
4.2 Salty water equation
The energy from the briny water in the desalination system is comparable to the total amount of energy that the briny water transferred to the glass by convective, radiative, and evaporative coefficients. So, the equilibrium formula for the brackish water can be expressed as:
The heat transfer coefficient between the basin and the water is specified by the following equation:
where \({G}_{r}\) , \(pr\) are Grashof and Prandtl numbers, respectively.
4.3 Solar still basin equation
The energy released from the basin can be defined as the summation of the energy transferred out by convection heat transfer between the basin and the salted water, energy loss to the air, and energy accumulation inside the SS absorber. This results in:
where \({H}_{b}\) is the total heat transfer coefficient in W/m 2 .K from the absorber plate to the air.
4.4 Output of the solar still
4.4.1 freshwater yield.
The output of the passive distilled system per hour is calculated by:
Where \(\lambda_{fg}\) and \({H}_{ew}\) are the latent heat and evaporation coefficient of heat transfer between the water and the glass, respectively, and can be assessed by:
where \({T}_{m}=\frac{{T}_{wat}+{T}_{gl}}{2}\) .
4.4.2 Energy efficiency
The conventional SS's efficiency is determined as (Abd Elbar et al., 2019 ; Elbar & Hassan, 2019 ; Hassan et al., 2019 ; Yousef et al., 2017 )
4.4.3 Exergy efficiency
The exergy analysis employs mass and energy conservation concepts (Abujazar et al., 2016 ). Exergy efficiency indeed represents the effectiveness of a system in converting input exergy into useful output exergy. It's commonly defined as the ratio of the exergy output of a process or system to the exergy input, expressed as a percentage, as shown in Sharshir et al. ( 2018 ); Abo-Elfadl et al., ( 2021 ).
4.5 Hybrid nano model
Adding nanoparticles with higher thermal conductivity to fluids, for example Titanium Oxide, Silicon oxide, Carbon Nanotubes, and Graphite, is one of the most substantial techniques for improving the heat transfer process (Hawwash et al., 2016 , 2018 , 2021 ).
4.5.1 The thermal conductivity
The most popular formula that defines the thermal conductivity for the nanofluid mainly depends on Maxwell–Garnett's model (Hassan & Harmand, 2013 ; Yang & Jiang, 2017 ) while in the case of the HNF, the property of conductivity is given by:
where \({K}_{bf}\) and \({K}_{hn}\) , are the thermal conductivities for the base fluid and hybrid nanofluid, respectively, in W/m.K, and \({\emptyset }_{hn}\) is the volume fraction for hybrid nanofluid such that \({\emptyset }_{hn}=\) \({\emptyset }_{\text{SiO}2}+{\emptyset }_{\text{TiO}2}\) .
4.5.2 The density
The density of the nanofluid can be described (Aminossadati & Ghasemi, 2009 ; Hassan, 2014 ) by:
where \({\rho }_{hn}\) describes the density of hybrid nanofluid, \({\rho }_{\text{SiO}2}\) , and \({\rho }_{\text{TiO}2}\) are densities for the Silicon oxide and the Titanium oxide, respectively, in kg/m 3 .
4.5.3 The specific heat
The specific heat of the hybrid nanoparticles (Bourantas & Loukopoulos, 2014 ) is presented by :
where \({{(C}_{p})}_{hn}\) is the specific heat of the hybrid nanofluid and \({C}_{p}\) is the specific heat of nanoparticles in J/kg.K.
4.5.4 The viscosity
The viscosity of the HNF is computed according to Batchelor's formula (Murshed et al., 2008 ) as follows:
4.6 Economic and exergoeconomic assessment
To accomplish the cost study of the distillation system without and with HNF, several parameters were considered, such as the initial cost of the solar still system (Ps), maintenance cost per year (AMC), salvage value per year (ASV), and the interest rate (i) was supposed to be equal 10%. The initial parameter in the cost analysis is the Capital recovery factor (CRF) which can be defined as (Gad et al., 2022 ):
Then, the total operational cost of the distillation system (UAC) per year is estimated by (Gad et al. 2022 ; Yousef et al., 2022 ):
where FAC is the fixed yearly cost that is calculated as the following:
and AMC is the maintenance cost per year, which is assumed to be 15% of the AMC:
The salvage value (S) of the solar still system is an important parameter and is considered by (Refat et al. 2019 ):
SFF and ASV can be assessed according to the subsequent formulations, respectively (Refat et al., 2019 ):
Finally, the price per litre for the solar still system can be computed by (Gad et al. 2022 ; Yousef et al., 2022 ):
where \({P}_{n}\) is the average annual of the produced water from the distillation system.
Due to the importance of the exergy analysis, which considers the real magnitude of thermodynamics and presents an actual measure of the system performance. So, in the current study, the exergoeconomic parameter ( \({R}_{exe}\) ) for all the suggested desalination systems can be assessed by the following relation:
4.7 Environmental and enviroeconomic evaluation
An environmental calculation had to be accomplished by computing the amount of CO 2 realized to the ambient to display the ecological and sustainability result of the MSS on the environment in addition to measuring its environmental superiority alongside the other traditional sources of energy. Therefore, the annual quantity of CO 2 mitigated in tons from the SS systems is set as (Yousef et al., 2022 ):
where \(\emptyset_{{{\text{co}}_{2} }}\) is the parameter of environmental and \({E}_{n}\) is the energy output from the solar still systems throughout the year. The enviroeconomic analysis is estimated as (Yousef et al., 2022 ):
where \({Z}_{{co}_{2}}\) is the parameter of enviro-economic and \({z}_{{co}_{2}}\) is the cost of carbon. The price was expected to be 14.5 $/ton.
5 Numerical solution
In this section, a numerical solution for computing the hourly changes in temperatures for the SS system by Atangana-Baleanu (AB) fractional operator is studied.
To rewrite the model given by Eqs. ( 4 – 8 ) in the sense of the Atangana-Baleanu fractional derivative, we need to replace the classical derivatives in the equations with Atangana-Baleanu fractional derivatives, then we have:
Now, using the following approximation (Atangana & Baleanu, 2016 ), the previous equation will be written as follows:
Use the above approximation. We get the following numerical scheme:
where \(w_{1 } \left( {n,\alpha ,\Delta t} \right) = \left[ {\frac{1 - \alpha }{{M\left( \alpha \right)}} - \frac{\alpha }{M\left( \alpha \right)\Gamma \left( \alpha \right)}\left( {\Delta t} \right)^{\alpha } \left( {\frac{{2\left( {n + 1} \right)^{\alpha } }}{\alpha } - \frac{{\left( {n + 1} \right)^{\alpha + 1} }}{\alpha + 1}} \right) - \frac{\alpha }{M\left( \alpha \right)\Gamma \left( \alpha \right)}\left( {\Delta t} \right)^{\alpha } \left( {\frac{{n^{\alpha } }}{\alpha } - \frac{{n^{\alpha + 1} }}{\alpha + 1}} \right)} \right],\)
and \(M\left(\alpha \right)\) is the Atangana-Baleanu function, where \(M\left(0\right)=M\left(1\right)=1.\) \(\Delta t\) is the time step.
6 Results and discussions
The results and discussion system are divided into two subsections: model validation and the influence of hybrid nanofluid on SS output.
6.1 Model validation
Figure 2 a–c displays a comparison between the hourly trends in glass temperature, freshwater production, and brackish water temperature in the SS basin using the experimental data from Hassan ( 2020 ) and the newly proposed fractional model. Based on the graph, the hourly variation in SS temperatures and SS freshwater production using the AB operator exhibits a remarkable consistency with the actual experimental data. The reason lies in the fact that the non-locality and non-singularity of kernel features, along with a noble memory influence, are considered by the fractional operator. The proposed fractional model simulates the SS behaviour effectively, with a percent of relative error for the greatest values of temperature of the glass, briny water temperature, and freshwater yield being 0.202%, 0.539%, and 0.757%, respectively.
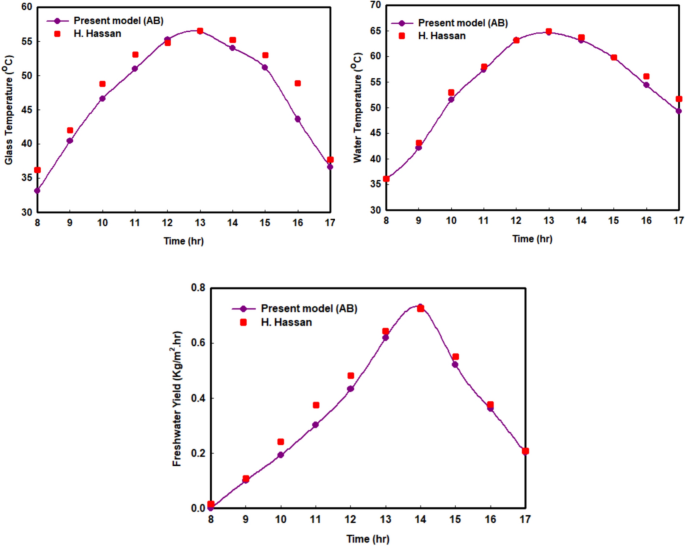
Variation of the solar still temperature and water productivity for the present model and Hassan ( 2020 ) experimental results
6.2 Influence of using hybrid nanoparticles
The CSS suffers from lower productivity and inadequate thermal performance primarily due to radiative and convective heat losses. However, the utilization of nanofluids has emerged as a promising technique to address these limitations. Nanofluids, created by incorporating nanometre-sized additives into a base fluid, exhibit superior thermophysical and optical properties, enabling enhanced heat transfer and decreased heat loss, resulting in increased freshwater production. By employing nanofluids, heat transfer coefficients are elevated, leading to improved performance of solar stills. Hybrid nanofluids, in particular, offer superior heat transfer capabilities in thermal processes, showcasing enhanced thermophysical properties compared to both conventional fluids and mono nanofluids. This research suggests a mixture of SiO 2 and TiO 2 nanoparticles, each at a volume fraction percentage of 0.025%, to be added to saline water. The scientific properties of these nanoparticles are explained in Table 1 .
6.2.1 Influence on Temperatures
The evaporation of salted water and the subsequent production of freshwater in the SS system is positively influenced by boosting the temperature of the briny water. Figure 3 a, b provide a comparison of the hourly variations of the distilled system temperatures during the winter season, both with and without the utilization of hybrid nanofluids. Similarly, Fig. 4 a, b display the same temperature results but for the summer period. These figures also include the atmosphere temperature and incident solar radiation during the measurement periods. Upon examining Figs. 3 and 4 , it is evident that the behaviour of the SS temperatures remains consistent when hybrid nanofluids are employed, resembling the case of using normal saline water fluid. Notably, a comparison of the water temperatures reveals that the presence of hybrid nanofluids leads to higher water temperatures, particularly during the summer season.
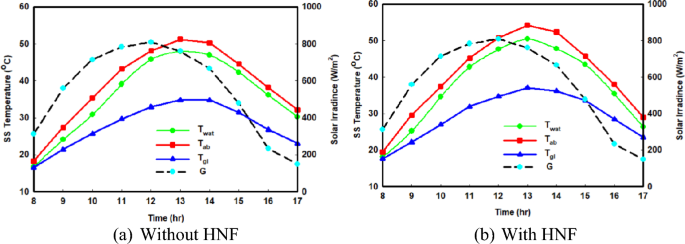
Hourly change in SS temperatures in cold climate conditions
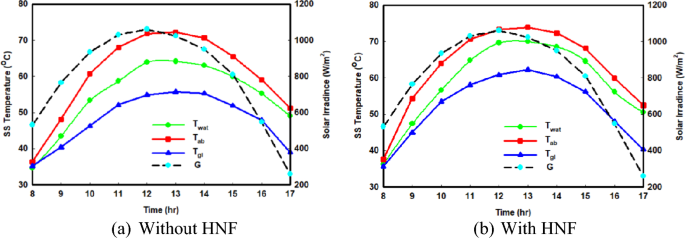
Hourly change in SS temperatures in hot climate conditions
The observed temperature increase can be attributed to the introduction of nanoparticles into the briny water, which enhances the thermophysical properties of the base fluid and consequently improves heat transfer, particularly in warm weather conditions, resulting in elevated temperature readings. For example, without the inclusion of hybrid nanoparticles, the maximum water temperatures in winter and summer peak at 47.91 °C and 64.15 °C, respectively. However, with the incorporation of hybrid nanofluids, these temperatures escalate to 50.42 °C and 69.99 °C, respectively, representing a noteworthy increase of 5.23% and 9.1% during the winter and summer seasons, respectively. Moreover, in hot climatic conditions, the peak glass temperature at midday rises from 55.73°C to 62.27°C following the introduction of the hybrid nanofluid, indicating a rise of 6.54°C in hot conditions.
6.2.2 Influence on productivity
The primary objective of the solar still (SS) system is to generate freshwater from saline water by harnessing solar radiation. Figure 5 a, b depict the hourly variations in freshwater productivity during winter and summer, both with and without the incorporation of hybrid nanofluids. It is evident from Fig. 5 that the amount of freshwater is higher in summer compared to winter, attributed to the greater solar insolation and, consequently, higher water temperature during the summer season, as previously explained. Furthermore, the use of hybrid nanoparticles leads to increased productivity in the solar still system as opposed to using only briny water. The maximum hourly values of freshwater production in winter and summer are 0.4253 kg/m 2 and 0.7317 kg/m 2 , respectively, for the system utilizing only saline water, while they reach 0.464 kg/m 2 h and 0.9772 kg/m 2 h, respectively, for the system incorporating hybrid nanoparticles.
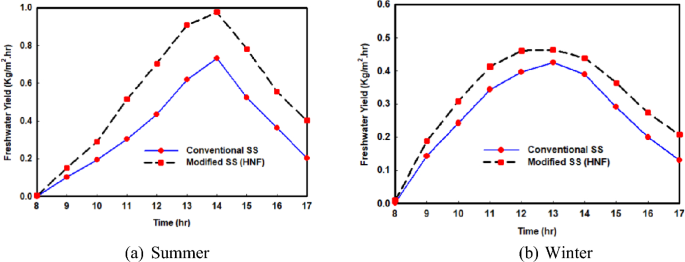
Variations of freshwater yield with and without hybrid nano
The observed enhancement in freshwater yield can be ascribed to the improved rates of condensation and evaporation of briny water facilitated by the application of hybrid nanofluids. Detailed results are presented in Table 2 , illustrating the average daily freshwater yield for the solar still system both without and with hybrid nanoparticles. During summer, the daily productivity with hybrid nanoparticles reaches 5.2922 kg/m 2 ⋅day, reflecting a notable increase of 27.2% compared to the still without nanoparticles. In winter, there is a commendable productivity increase of 21.7%, resulting in a daily yield of 3.1295 kg/m 2 ⋅day. Importantly, the amplified freshwater production due to nanoparticle usage is more prominent in summer, aligning with the heightened solar energy availability during that season. This amplification is consistent with the augmented impact of heat transfer enhancement with saline water through nanoparticle utilization, as elucidated in earlier discussions.
6.2.3 Influence on energy efficiency
During this analysis, we analyzed the energy efficiency of SS without and with HNF, both in winter and summer. Figure 6 a, b depict the varying energy efficiency over time, with results showing an increase in energy efficiency from 8 a.m. to close 2 p.m. followed by a gradual decline, similar to the productivity curve. As expected, solar efficiency was still lower in winter than in summer. Furthermore, the use of hybrid nanofluid had a substantial effect on the SS's efficiency, as it increased still productivity, ultimately resulting in higher energy efficiency.
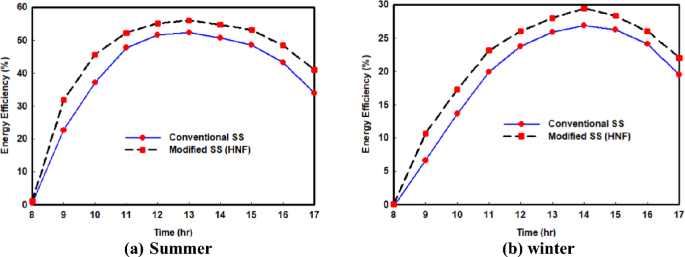
Variations of SS energy efficiency without and with hybrid nano
Results showed that the maximum energy efficiency with and without HNF in summer was 56.06% and 52.25%, respectively, while in winter, it was 29.45% and 26.9%, respectively. This represents an increase of 7.3% and 9.4% in summer and winter, respectively. In Table 3 , we present the average energy efficiency for the distilled system per day without and with HNF in winter and summer. The results demonstrate that in summer, the average efficiency increased by 13.1% when hybrid nano was used, achieving a maximum average efficiency of 43.95%. In comparison, the increase in efficiency in winter was 11.85%. The findings emphasize the potential of using hybrid nanofluid to enhance solar still efficiency.
6.2.4 Influence on exergy efficiency
This section delves into the influence of hybrid nanoparticles on exergy efficiency, a crucial aspect in engineering and thermodynamics for assessing and optimizing energy systems. Unlike traditional energy analysis focusing solely on energy quantity, exergy analysis studies both the quantity and property of energy, making it a powerful tool. Figure 7 a, b illustrate hourly fluctuations of exergy efficiency during summer and winter, respectively, comparing scenarios without and with hybrid nanoparticles. It's evident that exergy efficiency peaks around 1 p.m. and gradually declines. Notably, exergy efficiency tends to be lower than energy efficiency due to accounting for system irreversibilities and losses. Employing hybrid nanoparticles leads to higher exergy efficiency, with a more pronounced impact in summer due to increased solar energy availability. Peak exergy efficiency in summer rises from approximately 4.54% to nearly 5.09% with nanoparticle integration, while in winter, values increase from 3.68% to 4.11%. Table 4 presents average exergy efficiency per day, revealing a notable enhancement of approximately 20.14% in summer and 16.4% in winter with hybrid nanoparticles. This improvement stems from increased freshwater productivity facilitated by hybrid nanofluids, as previously demonstrated.
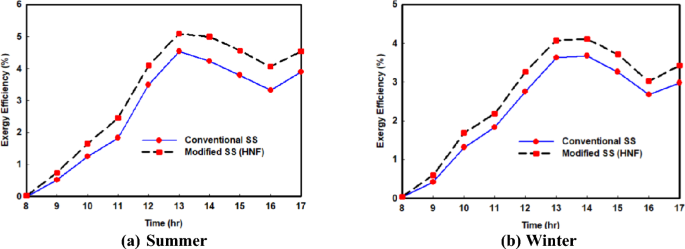
Variations of SS exergy efficiency with and without hybrid nano
6.3 Cost analysis for n = 10, i = 12%
The economic analysis, presented in Sect. 6.3 for n = 10 and i = 12%, meticulously evaluates the cost per litre (CPL) over a ten-year lifespan for the proposed SS systems during both winter and summer. Table 5 gives a detailed breakdown of the economic assessment. Notably, the incorporation of hybrid nanofluid in the modified solar still demonstrates a substantial reduction in CPL values compared to the CSS in both seasons. During summers, the CPL value for the modified SS with HNF is remarkably low at 0.0156 $/L, presenting a significant cost decrease of over 44% compared to the conventional still (0.0225 $/L). This reduction in CPL is attributed to the considerable enhancement in annual freshwater productivity resulting from the integration of hybrid nanofluid. The observed seasonal variation in CPL, with lower values during summer than winter, is primarily influenced by more favorable weather conditions and increased freshwater productivity in the former season.
6.4 Calculations of exergoeconomic parameters for n = 10, i = 12%
Exergoeconomic analysis plays a vital role in assessing the total efficiency and economic viability of energy systems. By combining the principles of thermodynamics and economics, engineers and policymakers enable to prioritize energy optimization strategies and make informed decisions regarding energy investments. The tabular representation of the calculation of R exe for both the MCC and CSS systems across various seasons, i.e., summer and winter, is depicted in Table 6 . On keenly observing the table, it becomes apparent that the MCC system, which utilizes a hybrid nanofluid, outperforms the CSS system in terms of R exe for both summer and winter. As an example, the R ex for the MCC and CSS systems in summer is calculated to be 6.605 (kWh/$) and 4.47 (kWh/$), respectively. This variance in R exe indicates that the former system outperforms the latter by approximately 47.7%. This improvement in R exe can be attributed to a minor increase in the UAC of the MCC system. However, its significant effect on the annual exergy output surpasses the decrease in UAC, resulting in a higher exergoeconomic parameter. Nonetheless, during winters, this increase of R exe with respect to the MSS system witnesses a slight reduction of 15.1%, as opposed to summers, due to a decrease in freshwater production enhancements.
6.5 Environmental and enviroeconomic analysis
Environmental and enviroeconomic analyses are of paramount importance when it comes to an understanding and mitigating CO 2 emissions, which are a major contributor to climate change. The environmental analysis focuses on assessing the mitigating CO 2 emissions. Enviroeconomic analysis, on the other hand, combines environmental factors with economic considerations to evaluate the costs and benefits associated with reducing CO 2 emissions. This analysis aids policymakers, businesses, and individuals in making informed decisions by highlighting the environmental and economic trade-offs and identifying the most effective and economically viable pathways to reduce CO 2 emissions. Table 7 provides a comparative overview of summer and winter environmental and enviro-economic evaluations conducted using energy and exergy methodologies. In terms of the environmental approach, the findings indicate that during summer, CSS and MSS mitigated 16.38 and 18.47 tons of CO 2 /year, respectively.
The exergy-environmental approach, on the other hand, found these values to be 1.68 and 2.62 tons CO 2 /year, respectively. These results reveal that MSS mitigated about 12.7% more CO 2 mitigation during summer when compared with CSS based on energy approaches. This trend is owing to the higher energy generated by MSS than the embodied energy integration of hybrid Nanofluid, resulting in increased CO 2 mitigation. Similarly, the findings based on the exergy approach suggest that MSS mitigated approximately 56.2% more CO 2 compared to CSS. Wintertime results show that compared to CSS, MSS mitigated approximately 29.6% and 22% more CO 2 based on energy and exergy approaches, respectively. Additionally, it is noteworthy that the addition of hybrid nanofluid to solar still significantly improved the enviro-economic parameter, as highlighted in Table 7 . In summer, the enviro-economic parameter for CSS and MSS was 237.6$ and 267.84$, respectively, According to the energy approach. The exergy-environmental approach produced equivalent values of 24.355$ and 38.05$, respectively.
7 Conclusion
The influence of titanium oxide (TiO 2 ) and silicon oxide (SiO 2 ) hybrid nanoparticles on the solar still performance (SS) is studied by applying a new numerical method based on the Atangana- Baleanu (AB) fractional operator. In addition, a comparison is conducted between the numerical findings of a fractional model and experimental data for the climate of Upper Egypt. The findings indicate that a fractional model corresponds to the actual experiment. It indicates that the error percentage in the extreme value of freshwater yield reaches approximately 0.757%. According to the fractional approach, the results demonstrate that the addition of hybrid nanoparticles to the solar still has a substantial influence on it in terms of boosting the quantity of freshwater production and enhancing both exergy and energy efficiency, with the winter impact being less pronounced than the summer impact. It has been shown that utilizing hybrid nanoparticles with the saltwater of CSS increases the production by approximately 27.2% in Summer and 21.7% in Winter. Additionally, its average daily efficiency increases to approximately 21.1% and 43.95% in winter and summer, respectively. In cold and hot climates, the average exergy efficiency of the SS per day is increased by 16.4% and 20.14%, respectively, when nanoparticles are added to saline water. An economic investigation showed that in the summer, SS with hybrid nanofluid reported a cost-per-liter reduction of over 44% compared to CSS. In terms of enviroeconomic analysis, findings indicate that SS with nanofluid mitigated approximately 12.7% more CO 2 during the summer than CSS based on energy approaches. Whereas, Wintertime results indicate that SS with nanofluid mitigated nearly 29.6% and 22% more CO 2 than CSS using energy and exergy approaches, respectively. For the future work, the effect of using HNF in active and passive SS should be investigated.
Our study has significant implications for key stakeholders. Policymakers can leverage hybrid nanofluids to improve solar still performance and promote sustainable freshwater production through supportive policies and funding. Academics can advance research in solar energy and nanofluid applications using our novel fractional model. Additionally, our findings offer valuable insights for researchers and businesses in renewable energy, highlighting the economic and environmental benefits of integrating hybrid nanofluids into solar still systems and suggesting avenues for commercialization and scale-up efforts.
Abbreviations
Atangana- Baleanu
Annual Maintenance Cost
Annual Salvage Value
Cost Per Liter
Conventional Solar Still
Capital Recovery Factor
Hybrid Nanofluid
Modified Solar Still
Sinking Fund Factor
Total Annual Cost
Area in m 2
Specific heat in J/kg.K
Solar irradiance in W/m 2
Heat transfer coefficient in W/m 2 .K
Evaporation coefficient of heat transfer
Thermal conductivity W/m.K
Capital Cost in $
Temperature in °C
Time in second
Wind velocity in m/s
Volume fractional
Density in kg/m 3
Order of fractional derivative ( \(0<\alpha \le 1)\)
The latent heat of vaporization
Absorber Plate
Evaporation
Abd Elbar, A. R., Yousef, M. S., & Hassan, H. (2019). Energy, exergy, exergoeconomic and enviroeconomic (4E) evaluation of a new integration of solar still with photovoltaic panel. Journal of Cleaner Production, 233 , 665–680. https://doi.org/10.1016/j.jclepro.2019.06.111
Article Google Scholar
Abdullah, A. S., Alawee, W. H., Mohammed, S. A., Majdi, A., Omara, Z. M., & Younes, M. M. (2023). Utilizing a single slope solar still with copper heating coil, external condenser, phase change material, along with internal and external reflectors—experimental study. Journal of Energy Storage, 63 , 106899. https://doi.org/10.1016/j.est.2023.106899
Abo-Elfadl, S., Yousef, M. S., & Hassan, H. (2021). Energy, exergy, and enviroeconomic assessment of double and single pass solar air heaters having a new design absorber. Process Safety and Environmental Protection, 149 , 451–464. https://doi.org/10.1016/j.psep.2020.11.020
Abujazar, M. S. S., Fatihah, S., Rakmi, A. R., & Shahrom, M. Z. (2016). The effects of design parameters on productivity performance of a solar still for seawater desalination: A review. Desalination, 385 , 178–193. https://doi.org/10.1016/j.desal.2016.02.025
Ahmed, M. M., Alshammari, F., Alqsair, U. F., Alhadri, M., Abdullah, A. S., & Elashmawy, M. (2022). Experimental study on the effect of the black wick on tubular solar still performance. Case Studies in Thermal Engineering, 38 , 102333. https://doi.org/10.1016/j.csite.2022.102333
Ajit, G. K., Malik, P., Garg, H., & Lamba, R. (2023). Thermophysical properties analysis of Al 2 O 3 , MgO and GO nanofluids with water for solar still. Materials Today: Proceedings . https://doi.org/10.1016/j.matpr.2023.06.383
Alsehli, M. (2022). Application of graphene nanoplatelet/platinum hybrid nanofluid in a novel design of solar still for improving water production and energy management. Sustainable Energy Technologies and Assessments, 53 , 102607. https://doi.org/10.1016/j.seta.2022.102607
Alshqirate, A., Awad, A. S., Al Alawin, A., & Essa, M. A. (2023). Experimental investigation of solar still productivity enhancement of distilled water by using natural fibers. Desalination, 553 , 116487. https://doi.org/10.1016/j.desal.2023.116487
Aly, W. I., Tolba, M. A., & Abdelmagied, M. (2023). Experimental investigation and performance evaluation of an oval tubular solar still with phase change material. Applied Thermal Engineering, 221 , 119628. https://doi.org/10.1016/j.applthermaleng.2022.119628
Aminossadati, S. M., & Ghasemi, B. (2009). Natural convection cooling of a localised heat source at the bottom of a nanofluid-filled enclosure. European Journal of Mechanics - B/Fluids, 28 , 630–640.
Angappan, G., Pandiaraj, S., Alrubaie, A. J., Muthusamy, S., Said, Z., Panchal, H., & Kabeel, A. E. (2023). Investigation on solar still with integration of solar cooker to enhance productivity: Experimental, exergy, and economic analysis. Journal of Water Process Engineering, 51 , 103470. https://doi.org/10.1016/j.jwpe.2022.103470
Asbik, M., Ansari, O., Bah, A., Zari, N., Mimet, A., & El-Ghetany, H. (2016). Exergy analysis of solar desalination still combined with heat storage system using phase change material (PCM). Desalination, 381 , 26–37. https://doi.org/10.1016/j.desal.2015.11.031
Atangana, A., & Baleanu, D. (2016). New fractional derivatives with nonlocal and non-singular kernel: Theory and application to heat transfer model. Thermal Science, 4 , 763–769.
Bait, O., & Si-Ameur, M. (2018). Enhanced heat and mass transfer in solar stills using nanofluids: A review. Solar Energy, 170 , 694–722. https://doi.org/10.1016/j.solener.2018.06.020
Bourantas, G. C., & Loukopoulos, V. C. (2014). Modeling the natural convective flow of micropolar nanofluids. International Journal of Heat and Mass Transfer, 68 , 35–41.
Chauhan, V. K., & Shukla, S. K. (2023). Performance analysis of prism shaped solar still using black phosphorus quantum dot material and lauric acid in composite climate: An experimental investigation. Solar Energy, 253 , 85–99. https://doi.org/10.1016/j.solener.2023.02.017
Chen, W., Zhou, J., Zhang, F., Li, X., & Guo, J. (2023). Stability study of low-cost carbon quantum dots nanofluids with saline water and their application investigation for the performance improvement of solar still. Diamond and Related Materials, 138 , 110194. https://doi.org/10.1016/j.diamond.2023.110194
Deniz, E., & Çınar, S. (2016). Energy, exergy, economic and environmental (4E) analysis of a solar desalination system with humidification-dehumidification. Energy Conversion and Management, 126 , 12–19. https://doi.org/10.1016/j.enconman.2016.07.064
Elbar, A., & Hassan, H. (2019). An experimental work on the performance of solar still incorporating with wind turbine and thermal energy storage unit. Desalination Water Treat, 165 , 24–34. https://doi.org/10.5004/dwt.2019.24492
Elbar, A. R. A., Yousef, M. S., & Hassan, H. (2019). Energy, exergy, exergoeconomic and enviroeconomic (4E) evaluation of a new integration of solar still with photovoltaic panel. Journal of Cleaner Production, 233 , 665–680. https://doi.org/10.1016/j.jclepro.2019.06.111
El-Gazar, E. F., Hassan, H., Rabia, S. I., & Zahra, W. K., (2023a). The thermal performance of the photovoltaic panel under the effect of air and water cooling: Modeling and simulation. In International Conference of Numerical Analysis and Applied Mathematics Icnaam 2021.
El-Gazar, E. F., Hassan, H., Rabia, S. I., Hu, C., & Zahra, W. K. (2023b). A new fractional Cattaneo model for enhancing the thermal performance of photovoltaic panels using heat spreader: Energy, exergy, economic and enviroeconomic (4E) analysis. Environmental Science and Pollution Research, 30 (48), 105840–105855. https://doi.org/10.1007/s11356-023-29654-8
El-Gazar, E. F., Hassan, H., Rabia, S. I., & Zahra, W. K. (2021a). Study of the impact of using hybrid nanofluid and saline water preheating on the performance of both integrated solar still and photovoltaic panel using fractional modeling. The European Physical Journal Plus, 136 , 1–37. https://doi.org/10.1140/epjp/s13360-021-01654-y
El-Gazar, E. F., Zahra, W. K., Hassan, H., & Rabia, S. I. (2021b). Fractional modeling for enhancing the thermal performance of conventional solar still using hybrid nanofluid: Energy and exergy analysis. Desalination, 503 , 114847. https://doi.org/10.1016/j.desal.2020.114847
Gad, R., Mahmoud, H., Ookawara, S., & Hassan, H. (2022). Energy, exergy, and economic assessment of thermal regulation of PV panel using hybrid heat pipe-phase change material cooling system. Journal of Cleaner Production, 364 , 132489. https://doi.org/10.1016/j.jclepro.2022.132489
Gaur, A., & Tiwari, G. N. (2014). Exergoeconomic and enviroeconomic analysis of photovoltaic modules of different solar cells. Journal of Solar Energy, 2014 , 1–8. https://doi.org/10.1155/2014/719424
Gupta, A., Gupta, A., Yadav, A. C., & Kumar, A. (2022). Performance analysis of single slope solar still under composite climate in India: Numerical simulation and thermal modeling approach. Materials Today: Proceedings, 63 , 699–705. https://doi.org/10.1016/j.matpr.2022.04.956
Hakim, A. R., Handoyo, W. T., & Wullandari, P. (2018). An energy and exergy analysis of photovoltaic system in Bantul Regency, Indonesia. Journal of Mechatronics, Electrical Power, and Vehicular Technology, 9 (1), 1–7. https://doi.org/10.14203/j.mev.2018.v9.1-7
Hameed, H. G. (2022). Experimentally evaluating the performance of single slope solar still with glass cover cooling and square cross-section hollow fins. Case Studies in Thermal Engineering, 40 , 102547. https://doi.org/10.1016/j.csite.2022.102547
Hassan, H. (2014). Heat transfer of Cu-water nanofluid in an enclosure with a heat sink and discrete heat source. European Journal of Mechanics, B/fluids, 45 , 72–83. https://doi.org/10.1016/j.euromechflu.2013.12.003
Hassan, H. (2020). Comparing the performance of passive and active double and single slope solar stills incorporated with parabolic trough collector via energy, exergy and productivity. Renewable Energy, 148 , 437–450. https://doi.org/10.1016/j.renene.2019.10.050
Hassan, H., Ahmed, M. S., Fathy, M., & Yousef, M. S. (2019). Impact of salty water medium and condenser on the performance of single acting solar still incorporated with parabolic trough collector. Desalination, 480 , 2020. https://doi.org/10.1016/j.desal.2020.114324
Hassan, H., & Harmand, S. (2013). 3D transient model of vapour chamber: Effect of nanofluids on its performance. Applied Thermal Engineering, 51 (1–2), 1191–1201. https://doi.org/10.1016/j.applthermaleng.2012.10.047
Hassan, H., & Osman, O. O. (2022). Novel dynamic simulation model and detailed performance evaluation of single slope solar still: Impact of side walls material. Solar Energy, 244 , 298–314. https://doi.org/10.1016/j.solener.2022.08.026
Hassan, H., Yousef, M. S., Ahmed, M. S., & Fathy, M. (2020). Energy, exergy, environmental, and economic analysis of natural and forced cooling of solar still with porous media. Environmental Science and Pollution Research, 27 (30), 38221–38240. https://doi.org/10.1007/s11356-020-09995-4
Hawwash, A. A., Abdel-Rahman, A. K., & Nada, S. A. (2016) Experimental study of alumina nanofluids effects on thermal performance efficiency of flat plate solar collectors. In 5th Annual International Conference on Sustainable Energy and Environmental Sciences (SEES 2016) , Global Science & technology Forum ( GSTF ). https://doi.org/10.5176/2251-189X_SEES16.29 .
Hawwash, A. A., Mori, S., El Feky, K., & Hassan, H. (2019) Numerical study for open reactor design using salt hydrate. In IOP Conference Series: Earth and Environmental Science , Institute of Physics Publishing, https://doi.org/10.1088/1755-1315/322/1/012021 .
Hawwash, A. A., Ahamed, M., Nada, S. A., Radwan, A., & Abdel-Rahman, A. K. (2021). Thermal analysis of flat plate solar collector using different nanofluids and nanoparticles percentages. IEEE Access, 9 , 52053–52066. https://doi.org/10.1109/ACCESS.2021.3060004
Hawwash, A. A., Mori, S., & Hassan, H. (2023). An experimental investigation on the performance of designed closed reactor system on the thermochemical heat storage of magnesium chloride hexahydrate. Experimental Heat Transfer . https://doi.org/10.1080/08916152.2023.2240807
Hawwash, A. A., Rahman, A. K. A., Nada, S. A., & Ookawara, S. (2018). Numerical investigation and experimental verification of performance enhancement of flat plate solar collector using nanofluids. Applied Thermal Engineering, 130 , 363–374. https://doi.org/10.1016/j.applthermaleng.2017.11.027
HM Hussen MM Younes WH Alawee AS Abdullah SA Mohammed T Atteya ZM Faheem Abbas Omara 2023 An experimental comparison study between four different designs of solar stills Case Studies in Thermal Engineering 44 102841 https://doi.org/10.1016/j.csite.2023.102841
Ibrahim, A. G. M., Rashad, A. M., & Dincer, I. (2017). Exergoeconomic analysis for cost optimization of a solar distillation system. Solar Energy, 151 , 22–32. https://doi.org/10.1016/j.solener.2017.05.020
Jafarkazemi, F., & Ahmadifard, E. (2013). Energetic and exergetic evaluation of flat plate solar collectors. Renewable Energy, 56 , 55–63. https://doi.org/10.1016/j.renene.2012.10.031
Joshi, P., & Tiwari, G. N. (2018). Energy matrices, exergo-economic and enviro-economic analysis of an active single slope solar still integrated with a heat exchanger: A comparative study. Desalination, 443 , 85–98. https://doi.org/10.1016/j.desal.2018.05.012
Kabeel, A. E., et al. (2019). Effect of water depth on a novel absorber plate of pyramid solar still coated with TiO 2 nano black paint. Journal of Cleaner Production, 213 , 185–191. https://doi.org/10.1016/j.jclepro.2018.12.185
Kaviti, A. K., Akkala, S. R., Ali, M. A., Anusha, P., & Sikarwar, V. S. (2023). Performance Improvement of Solar Desalination System Based on CeO2-MWCNT Hybrid Nanofluid. Sustainability, 15 (5), 4268. https://doi.org/10.3390/su15054268
Keshtkar, M., Eslami, M., & Jafarpur, K. (2020). Effect of design parameters on performance of passive basin solar stills considering instantaneous ambient conditions: A transient CFD modeling. Solar Energy, 201 , 884–907. https://doi.org/10.1016/j.solener.2020.03.068
Kianifar, A., Heris, S. Z., & Mahian, O. (2012). Exergy and economic analysis of a pyramid-shaped solar water purification system: Active and passive cases. Energy, 38 (1), 31–36. https://doi.org/10.1016/j.energy.2011.12.046
R Kumar J Chanda AH Elsheikh B Ongar Y Khidolda S PraveenKumar S Hitesh Panchal Shanmugan 2023 Performance improvement of single and double effect solar stills with silver balls/nanofluids for bioactivation: An experimental analysis Solar Energy 259 452 463 https://doi.org/10.1016/j.solener.2023.05.012
Kumar, N. M., Subramaniam, U., Mathew, M., Ajitha, A., & Almakhles, D. J. (2020). Exergy analysis of thin-film solar PV module in ground-mount, floating and submerged installation methods. Case Studies in Thermal Engineering, 21 , 100686. https://doi.org/10.1016/j.csite.2020.100686
Kumaravel, S., Nagaraj, M., & Bharathiraja, G. (2022). Experimental investigation on the performance analysis of blue metal stones and pebble stones as thermal energy storage materials in single slope solar still. Mater Today Proc . https://doi.org/10.1016/j.matpr.2022.11.100
Lisboa, A. A. V., Segurado, R., & Mendes, M. A. A. (2022). Solar still performance for small-scale and low-cost seawater desalination: Model-based analysis and water yield enhancement techniques. Solar Energy, 238 , 341–362. https://doi.org/10.1016/j.solener.2022.04.007
Maatki, C. (2022). Heat transfer enhancement using CNT-water nanofluids and two stages of seawater supply in the triangular solar still. Case Studies in Thermal Engineering, 30 , 101753. https://doi.org/10.1016/j.csite.2021.101753
P Manoj Kumar PT Saravanakumar Atul Sarojwal D Rajasekaran Saminathan SJ Harikrishna RA Prasanth Pranav 2023 Experimental investigations on the performance of a single slope solar still with thermal energy storage Materials Today: Proceedings https://doi.org/10.1016/j.matpr.2022.12.221
Mittal, G. (2021). An unsteady CFD modelling of a single slope solar still. Materials Today: Proceedings, 46 , 10991–10995. https://doi.org/10.1016/j.matpr.2021.02.090
Modi, K. V., & Gamit, A. R. (2022). Investigation on performance of square pyramid solar still using nanofluid and thermal energy storage material: An experimental and theoretical study. Journal of Cleaner Production, 381 , 135115. https://doi.org/10.1016/j.jclepro.2022.135115
Moustafa, E. B., Hammad, A. H., & Elsheikh, A. H. (2022). A new optimized artificial neural network model to predict thermal efficiency and water yield of tubular solar still. Case Studies in Thermal Engineering, 30 , 101750. https://doi.org/10.1016/j.csite.2021.101750
Murshed, S. M., Leong, K. C., & Yang, C. (2008). Thermophysical and electrokinetic properties of nanofluids—A critical review. Applied Thermal Engineering, 28 , 2109–2152.
Mustafa, J., Abdullah, M. M., Husain, S., Alqaed, S., Malekshah, E. H., & Sharifpur, M. (2023). A two-phase analysis of the use of water-aluminum nanofluid in a solar still with a layer of phase change materials. Eng Anal Bound Elem, 152 , 627–636. https://doi.org/10.1016/j.enganabound.2023.04.030
Obembe, A. D., Hossain, M. E., & Abu-Khamsin, S. A. (2017). Variable-order derivative time fractional diffusion model for heterogeneous porous media. Journal of Petroleum Science and Engineering, 152 , 391–405. https://doi.org/10.1016/j.petrol.2017.03.015
Pal, P., Dev, R., Singh, D., & Ahsan, A. (2018). Energy matrices, exergoeconomic and enviroeconomic analysis of modified multi–wick basin type double slope solar still. Desalination, 447 , 55–73. https://doi.org/10.1016/j.desal.2018.09.006
Rabbi, H. M. F., & Sahin, A. Z. (2021). Performance improvement of solar still by using hybrid nanofluids. Journal of Thermal Analysis and Calorimetry, 143 (2), 1345–1360. https://doi.org/10.1007/s10973-020-10155-6
Ranjan, K. R., Kaushik, S. C., & Panwar, N. L. (2016). Energy and exergy analysis of passive solar distillation systems. International Journal of Low-Carbon Technologies, 11 (2), 211–221. https://doi.org/10.1093/ijlct/ctt069
Rashidi, S., Bovand, M., Rahbar, N., & Esfahani, J. A. (2018). Steps optimization and productivity enhancement in a nanofluid cascade solar still. Renewable Energy, 118 , 536–545. https://doi.org/10.1016/j.renene.2017.11.048
Rashidi, S., Esfahani, J. A., & Rashidi, A. (2017). A review on the applications of porous materials in solar energy systems. Renewable and Sustainable Energy Reviews, 73 , 1198–1210. https://doi.org/10.1016/j.rser.2017.02.028
Refat, A., Elbar, A., Yousef, M. S., & Hassan, H. (2019). of a new integration of solar still with photovoltaic panel. Journal of Cleaner Production, 233 , 665–680. https://doi.org/10.1016/j.jclepro.2019.06.111
Sahota, L., Arora, S., Singh, H. P., & Sahoo, G. (2020). Thermo-physical characteristics of passive double slope solar still loaded with mwcnts and al2o3-water based nanofluid. Materials Today: Proceedings, 32 , 344–349. https://doi.org/10.1016/j.matpr.2020.01.600
Sahota, L., & Shyam, G. N. T. (2017). Energy matrices, enviroeconomic and exergoeconomic analysis of passive double slope solar still with water based nanofluids. Desalination, 409 , 66–79. https://doi.org/10.1016/j.desal.2017.01.012
Sahu, R., & Tiwari, A. C. (2024). Performance enhancement of single slope solar still using nanofluids at different water depth. Desalination and Water Treatment, 317 , 100046. https://doi.org/10.1016/j.dwt.2024.100046
Sharshir, S. W., Guilong Peng, A. H., Elsheikh, E. M. A., Edreis, M. A., Eltawil, T. A., Kabeel, A. E., Zang, J., & Yang, N. (2018). Energy and exergy analysis of solar stills with micro/nano particles: A comparative study. Energy Conversion and Management, 177 , 363–375. https://doi.org/10.1016/j.enconman.2018.09.074
Sharshir, S. W., Kandeal, A. W., Algazzar, A. M., Eldesoukey, A., El-Samadony, M. O. A., & Hussien, A. A. (2022). 4-E analysis of pyramid solar still augmented with external condenser, evacuated tubes, nanofluid and ultrasonic foggers: A comprehensive study. Process Safety and Environmental Protection, 164 , 408–417. https://doi.org/10.1016/j.psep.2022.06.026
Shoeibi, S., Kargarsharifabad, H., Rahbar, N., Ahmadi, G., & Safaei, M. R. (2022). Performance evaluation of a solar still using hybrid nanofluid glass cooling-CFD simulation and environmental analysis. Sustainable Energy Technologies and Assessments, 49 , 101728. https://doi.org/10.1016/j.seta.2021.101728
Singh, D. B. (2019). Exergo-economic, enviro-economic and productivity analyses of N identical evacuated tubular collectors integrated double slope solar still. Applied Thermal Engineering, 148 , 96–104. https://doi.org/10.1016/j.applthermaleng.2018.10.127
Singh, D. B., & Tiwari, G. N. (2017). Exergoeconomic, enviroeconomic and productivity analyses of basin type solar stills by incorporating N identical PVT compound parabolic concentrator collectors: A comparative study. Energy Conversion and Management, 135 , 129–147. https://doi.org/10.1016/j.enconman.2016.12.039
Sun, H., Chang, A., Zhang, Y., & Chen, W. (2019). A review on variable-order fractional differential equations: Mathematical foundations, physical models, numerical methods and applications. Fract Calc Appl Anal, 22 (1), 27–59. https://doi.org/10.1515/fca-2019-0003
Teodoro, G. S., Machado, J. T., & De Oliveira, E. C. (2019). A review of definitions of fractional derivatives and other operators. Journal of Computational Physics, 388 , 195–208. https://doi.org/10.1016/j.jcp.2019.03.008
Toosi, S. S. A., Goshayeshi, H. R., Zahmatkesh, I., & Nejati, V. (2023). Experimental assessment of new designed stepped solar still with Fe3O4+ graphene oxide+ paraffin as nanofluid under constant magnetic field. Journal of Energy Storage, 62 , 106795. https://doi.org/10.1016/j.est.2023.106795
Yang, L., & Jiang, W. (2017). A new thermal conductivity model for nanorod-based nanofluids. Applied Thermal Engineering, 114 , 287–299.
Yousef, M. S., & Hassan, H. (2019). Energetic and exergetic performance assessment of the inclusion of phase change materials (PCM) in a solar distillation system. Energy conversion and management, 179 , 349–361. https://doi.org/10.1016/j.enconman.2018.10.078
Yousef, M. S., Hassan, H., Ahmed, M., & Ookawara, S. (2017). Energy and exergy analysis of single slope passive solar still under Egyptian climate conditions. Energy Procedia, 141 (00), 18–23. https://doi.org/10.1016/j.egypro.2017.11.005
Yousef, M. S., Sharaf, M., & Huzayyin, A. S. (2022). Energy, exergy, economic, and enviroeconomic assessment of a photovoltaic module incorporated with a paraffin-metal foam composite: An experimental study. Energy, 238 , 121807. https://doi.org/10.1016/j.energy.2021.121807
Žecová, M., & Terpák, J. (2015). Heat conduction modeling by using fractional-order derivatives. Applied Mathematics and Computation, 257 , 365–373. https://doi.org/10.1016/j.amc.2014.12.136
Download references
Acknowledgements
The authors are pleased to acknowledge Benha University for supporting the present work.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Basic Science Department, Benha Faculty of Engineering, Benha University, Benha, Egypt
E. F. El-Gazar & Mahmoud A. Khattab
Department of Thermal and Fluid Engineering, Universidad Carlos III de Madrid, Av. de La Universidad, 30, 28911, Leganés, Madrid, Spain
Mohamed S. Yousef
Mechanical Engineering Department, Faculty of Engineering, Benha University, Benha, Egypt
Abdelrahman M. Elshaer, T. A. Mouneer & A. A. Hawwash
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to E. F. El-Gazar .
Ethics declarations
Conflicts of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
El-Gazar, E.F., Yousef, M.S., Elshaer, A.M. et al. Enhancing solar still performance with hybrid nanofluid: a comprehensive assessment of energy, exergy, economics, and environmental impact using a novel fractional model. Environ Dev Sustain (2024). https://doi.org/10.1007/s10668-024-05304-y
Download citation
Received : 23 August 2023
Accepted : 05 August 2024
Published : 24 August 2024
DOI : https://doi.org/10.1007/s10668-024-05304-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Atangana-Baleanu
- Fractional differential equation
- Solar still
- Hybrid nanoparticles
- Economical assessment
- Environmental assessment
- Find a journal
- Publish with us
- Track your research
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search

Conservation of Mass - NEW AQA GCSE
Subject: Chemistry
Age range: 14-16
Resource type: Worksheet/Activity
Last updated
18 July 2024
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

Creative Commons "Sharealike"
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
pamalik7860
Empty reply does not make any sense for the end user
oliver_day95
Lovely presentation but beware the balancing equations worksheet - it contains some incomplete formula and reactions which I believe do not actually exist toward the bottom (PH5 + O2 --- P2O5 + H2O) XD
victoria_josiah
Thank you for sharing
jdwooldridge17
Sarahannmorley.
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:

COMMENTS
Subject: Chemistry. Age range: 14-16. Resource type: Other. File previews. pptx, 688.03 KB. Combustion / burning Magnesium (Mg) ribbon and weighing to find the empirical formula of Magnesium Oxide (MgO). Includes worksheet at end of PPT to make calculation easier. Creative Commons "Sharealike". to let us know if it violates our terms and ...
The demonstration is then carried out, students should record in their books the mass of the magnesium before burning and then the mass of the magnesium oxide after burning, from this they can deduce the mass of the oxygen needed to burn the magnesium. Students can then check their predictions to see if they were correct or not.
This should allow them to calculate the mass of the mass of the magnesium (mass 2 - mass 1) and the mass of the product (mass 3 - mass 1). They could also calculate the increase in mass (mass 3 - mass 2), which corresponds to the mass of oxygen. The equation is: Magnesium + oxygen → magnesium oxide. 2Mg + O 2 → 2MgO.
Conservation of mass. Subject: Chemistry. Age range: 14-16. Resource type: Worksheet/Activity. File previews. pptx, 72.12 KB. pptx, 1.43 MB. Lesson on conservation of mass and some balancing work. Includes a practical sheet for the burning magnesium with oxygen experiment where students work out the change in mass due to added oxygen.
Disposal. Scrap the magnesium oxide left in the crucible using a spatula and dispose of as general waste. Crucibles can be soaked for a few hours or overnight in 0.5 mol dm-3 hydrochloric acid solution if any solid remains at the bottom, rinse thoroughly using distilled water. Porcelain crucibles tend to crack easily if re-used, consider using ...
Procedure for the reaction of magnesium with oxygen. 1. Take a piece of magnesium about 10-15 cm long. Twist it into a loose coil. 2. Put the magnesium inside the crucible and place the crucible with the lid on a mass balance. Record the total mass of the magnesium, crucible and lid. 3.
2 Mg(s) + O2(g) → 2 MgO(s) In this experiment, an analytical balance is used to determine the mass of magnesium ribbon and magnesium oxide. The mass of oxygen that reacts with magnesium is calculated by the difference between these two. The number of moles of each element and molar ratio in the product of magnesium oxide can then be calculated.
I react a known mass of magnesium in a crucible and measure the change in mass, due to oxygen atoms joining with the magnesium atoms and forming magnesium ox...
Burning magnesium to show the law of conservation of mass 8fc2 Periodic Table lesson 4. KS3 science AQA.
Lesson follows Exploring Science textbook in content covering oxidation, metal oxides, law of conservation of mass and investigating mass of magnesium oxide practical. All resources needed included, no licenses required. 1 practical set up needed. ~1 hour content
The law of conservation of mass states that mass in a closed system will not change before and after a chemical reaction. ... This is because as it burns it combines with oxygen in the air to form iron oxide. The addition of the oxygen atoms causes an increase in mass. It's a great experiment to get students thinking about chemical reactions ...
The law of conservation of mass states that no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants. AQA Combined science: Synergy. 4.5 Building blocks for understanding. 4.5.2 Chemical quantities. 4.5.2.2 Conservation of mass. Recall and use the law of conservation of mass.
Chemistry 1210 Experiment 4: The Law of Conservation of Mass. Objective. In this experiment you will burn a sample of magnesium metal in air and demonstrate the. increase in mass due to formation of magnesium oxide, MgO. Then using the reaction. stoichiometry you will verify the Law of Conservation of Mass. Introduction.
n of MassWorksheetBackgroundAntoine Lavoisier was a French chemist who did most. f his work between 1772-1786. He built a magnificent laboratory in Paris, France and invited scientists from arou. the world to come and visit. Lavoisier conducted n. erous controlled experiments. He published two textbooks that helped organize chemistry.
Magnesium Oxide Magnesium is a light, silver grey metal. Oxygen is a colourless, odourless gas. Magnesium Oxide is a white po. International; Resources; ... Burning Magnesium Experiment. Subject: Chemistry. Age range: 11-14. Resource type: Worksheet/Activity. missmunchie. 3.63 643 reviews. ... Tes Global Ltd is registered in England (Company No ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright ...
The correct formula for magnesium oxide is MgO, a 1.0 to 1.0 ratio. But sometimes in this experiment the ratio of Mg to O comes out too low. (Example: 0.9 Mg to 1.0 O) In that case, it means that there was too much oxygen relative to the mass of magnesium. At other times it comes out that the ratio is too large.
In the present study, the thermal performance of a modified solar still (MSS) system coupled with hybrid nanofluid (HNF) of titanium oxide (TiO2) and silicon oxide (SiO2) has been investigated theoretically based on energetic, exergetic, economic, and enviroeconomic assessment. The model of the MSS has been introduced using a new numerical technique of the Atangana-Baleanu fractional ...
Age range: 14-16. Resource type: Worksheet/Activity. File previews. ppt, 303 KB. doc, 107 KB. doc, 184.5 KB. doc, 222 KB. Power point about conservation of mass with 2 practicals to demonstrate how mass is conserved. Worksheet can be used as a plenary / starter.
whole lesson including AfL strategies on conservation of mass/ balancing equations. Creative Commons "Sharealike" Reviews. 4.5 Something went wrong, please try again later. pamalik7860. a year ago. report. 4. thank you ... Tes Global Ltd is registered in England (Company No 02017289) with its registered office at Building 3, St Paul's Place ...