An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Trends in mechanical ventilation: are we ventilating our patients in the best possible way?
Raffaele l dellaca’, chiara veneroni, ramon farre’.
- Author information
- Copyright and License information
Email: [email protected]
Breathe articles are open access and distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0 .
This review addresses how the combination of physiology, medicine and engineering principles contributed to the development and advancement of mechanical ventilation, emphasising the most urgent needs for improvement and the most promising directions of future development.
Several aspects of mechanical ventilation are introduced, highlighting on one side the importance of interdisciplinary research for further development and, on the other, the importance of training physicians sufficiently on the technological aspects of modern devices to exploit properly the great complexity and potentials of this treatment.
Educational aims
To learn how mechanical ventilation developed in recent decades and to provide a better understanding of the actual technology and practice.
To learn how and why interdisciplinary research and competences are necessary for providing the best ventilation treatment to patients.
To understand which are the most relevant technical limitations in modern mechanical ventilators that can affect their performance in delivery of the treatment.
To better understand and classify ventilation modes.
To learn the classification, benefits, drawbacks and future perspectives of automatic ventilation tailoring algorithms.
Short abstract
Physiology, medicine and engineering principles have contributed to the advancement of mechanical ventilation http://ow.ly/Fjlb30bFpLl
Introduction
Even though the concept of mechanical ventilation dates back to the 14th century with V esalius [ 1 ], it is only in the last century that it has been widely introduced into routine clinical practice. Since their initial appearance, mechanical ventilators have become more sophisticated and expanded their application from the intensive care unit (ICU) to the respiratory medicine ward and even to patients’ homes for long-term treatments. This was the result of combining the advances in our understanding of respiratory physiology, pathophysiology and clinical management of patients together with technological progress in mechanical, electronic and biomedical engineering.
Nowadays, this evolution is still rapid, with new devices and an increased number of ventilation modes and strategies being introduced to improve outcomes, patient–ventilator interactions and patient care. Engineering has played and is still playing a relevant role in this process, not only in improving the technical performance of the ventilators but also in contributing to a better understanding of respiratory physiology and pathophysiology, and of how different ventilation strategies interact with the respiratory system.
This review addresses how the combination of physiology, medicine and engineering principles has contributed to the development and advancement of mechanical ventilation, highlighting the open issues and the most urgent needs for improvement with a view on the most promising directions of future development.
Evolution of mechanical ventilation
The use of mechanical tools to assist ventilation dates back to the late 19th century, when devices able to apply an alternating subatmospheric pressure around the body were used to restore ventilation by expanding the chest wall of patients [ 2 ]. However, it was only after the introduction of positive-pressure ventilation during the reappearance of poliomyelitis in the 1950s, when Bjorn Ibsen demonstrated a dramatic reduction of mortality in patients manually ventilated by tracheostomy, that mechanical ventilation started to be widespread [ 3 ].
The first positive-pressure mechanical ventilators became available in 1940. Though characterised by a significant degree of sophistication, they were able to deliver only a pre-set tidal volume at a given respiratory rate (volume-control ventilation mode) with no or very limited capability to monitor ventilation variables ( figure 1a ).
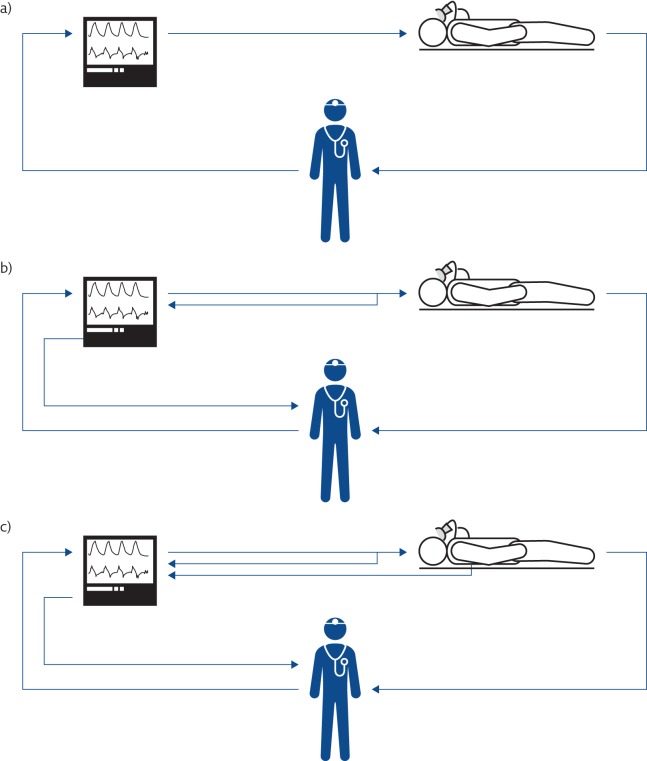
Evolution of the concept of mechanical ventilation. a) The first mechanical ventilators were not equipped with sensors. b) Mechanical ventilators monitor all the ventilation parameters, allowing both closed-loop control of the generated waveform and providing information to the clinicians. c) Mechanical ventilators monitor the condition of the patients and automatically adjust the ventilatory parameters on the basis of patients’ needs.
At that time, respiratory physiology had already established its foundations and was rapidly growing. The application of mathematical modelling to describe the relationships between flow and pressure (a wonderful example of what today would be considered a biomedical engineering approach), which marked the dawn of the mechanics of breathing, was introduced by D ixon and B rodie [ 4 ] in 1903, who modelled the lung as resistance and compliance. R ohrer [ 5 ] introduced a relevant simplification by considering only one pressure across the lung and one across the chest wall in 1915 (modelling confirmed by M ead et al. [ 6 ] in 1970), improving our capability to describe and understanding the mechanics of the respiratory system during both physiological and artificial breaths. R ahn et al. [ 7 ] introduced pressure–volume diagrams of the lung and thorax, and the concept of relaxation curves, in 1946, creating the background for the development of respiratory energetics. These and other studies constituted the physiological background that lead to the introduction of positive-pressure ventilation into clinical practice.
The main objective of mechanical ventilation was originally focused on restoring patients’ ventilation. This concept expanded as the other variables determining gas exchange were understood. A shbaugh et al. [ 8 ] first described acute respiratory distress syndrome (ARDS) in 1967 and identified the positive end-expiratory pressure (PEEP) as “most helpful in combating atelectasis and hypoxæmia”. During the same period, animal studies showing oxygen toxicity when a high inspiratory oxygen fraction ( F IO 2 ) was used suggested increasing ventilation instead of F IO 2 to treat hypoxaemia, leading to the (ab)use of increased tidal volume ( V T ).
From the early 1970s, ventilators benefited from the progresses in electronics, and started incorporating more advanced monitoring of flow and pressure variables ( figure 1b ). Improvements in monitoring also allowed the possibility of using real-time variables to control the action of the machine, with the intermittent mandatory ventilation mode opening the development of assisted mechanical ventilation as a way to manage the weaning of patients from periods of volume-controlled ventilation [ 9 ].
In the meantime, our understanding of the mechanics of breathing further improved thanks to the introduction of the concept of dynamic compression in airways during forced expiration by Fry, Hyatt and co-workers [ 10 ]; three-dimensional analysis of pressure, flow and volume curves [ 10 , 11 ]; and a better understanding of chest wall mechanics from Campbell’s diagram for the partitioning of elastic and resistive work [ 12 ], and the two-compartment model of the chest wall by K onno and M ead [ 13 ].
In 1974, W ebb and T ierney [ 14 ] demonstrated in animal models that ventilation with high distending pressures was leading per se to severe or even fatal pulmonary oedema, marking the beginning of a decades-long research effort aimed at understanding the adverse effects of mechanical ventilation and the mechanisms involved, and identifying appropriate strategies for preventing ventilation-induced lung injury (VILI), which despite the huge progress made so far, is still an open issue. Since then, major attention has been given to identifying the optimal ventilation strategy as a compromise between normalisation of blood gases and avoiding the development of VILI, resulting in the introduction of improved technologies for monitoring ventilation and lung conditions and the introduction of new advanced ventilation modes.
This progress was also possible thanks to the introduction of microprocessors in mechanical ventilators, starting in the early 1980s. Microprocessors are silicon electronic circuits able to execute programs; therefore, they can provide very complex functions that can be easily changed by selecting different programs, allowing a modern mechanical ventilator to rapidly modify its behaviour ( e.g. changing from a volume-control to a pressure-control or a pressure-assist ventilation mode simply by selecting different control algorithms executed by the device). In addition, microprocessors allow the implementation of sophisticated signal processing of the measurements provided by sensors, leading not only to better measurement accuracy and better rejection of noise and artefacts, but also to the generation of new information by appropriately computing and integrating several variables. Moreover, this expanded information can be used by the ventilator itself to optimise the ventilation parameters on the basis of patient needs, creating the so-called “closed-loop” ventilation strategies or intelligent or smart ventilation modes ( figure 1c ).
The mechanical ventilator: the challenge of delivering accurate ventilation
Once a ventilation strategy is defined, the ventilator should deliver it to the patient in the most accurate way. To achieve this, the machine must sense all variables that define the breathing pattern with high accuracy and adjust its action in real time. Modern ventilators achieve this by combining cutting-edge technology of actuators, sensors and digital electronics together with sophisticated data processing algorithms. The basic components of a typical mechanical ventilator are shown in figure 2 .
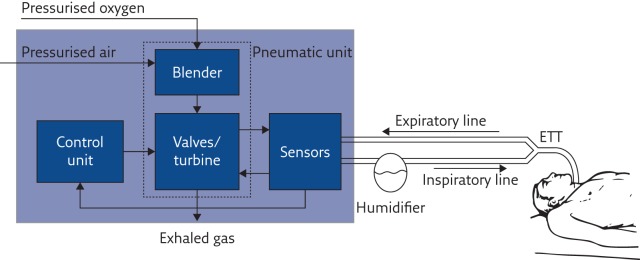
Basic structure and main functional components of a mechanical ventilator. ETT: endotracheal tube.
Pneumatic unit
The pressure source provides the energy required to overcome the elastic and resistive load imposed by the patient’s respiratory system, and it is used to reduce their work of breathing. The pressurised air is mixed with the appropriate amount of oxygen by the blender, and delivered to the patient by fast valves that modulate the amount of gas flowing to and from the patient. Thanks to the recent progress in electronics and electric motors, instead of using valves, in some modern ventilators a fast-response, brushless-driven turbine acts as a variable pressurised air source, making the device independent of centralised medical compressed air distribution but still providing good performance [ 15 ].
In modern mechanical ventilators, all relevant ventilation variables (pressure, flow and F IO 2 ) are measured by appropriate sensors that provide information to the control unit in order to adjust, in real time, the valves/turbines for delivering the desired ventilation mode.
Modern pressure transducers produce stable, precise and fast measurement of pressure but, despite this, pressure variables provided by ventilators are at times still outside the tolerance range, even in absence of leaks [ 16 ]. Aimed at measuring an extensive variable, flow sensors (that also provide volume data by computing the time integral of flow) must be placed in series with the patient in the breathing circuit and, therefore, are exposed to humidity, condensation and the patient’s exhalation, making them potential sites of cross-contamination. Moreover, flow meters must guarantee a wide operating range of measurements, high accuracy and stability, adequate dynamic response, robustness to changes in gas composition, temperature and vapour condensation, and at the same time, impose a low pneumatic resistance and dead space on the patient.
The need to improve flow measurement technology is highlighted by the several comparative publications on performance of mechanical ventilators that quite often identify the delivered volume as the variable showing the lowest accuracy and repeatability [ 16 – 18 ].
The most common approaches to measuring flow include variable-orifice sensors, pneumotachographs or hot-wire anemometers. Promising technologies for the future are micromachined and fibreoptic-based flow meters [ 19 ]. In particular, thermal flow sensors micromachined on a single silicon chip are already available and used in some mechanical ventilators. They have the advantages of presenting high sensitivity and accuracy, a wide measurement range, fast response, low temperature and gas composition sensitivity, low dead space and resistance [ 20 ], and the production cost could lead, in the future, to the availability of pre-calibrated disposable devices.
Ventilator circuit
Few studies have addressed the impact of ventilator circuits on overall performance in adult ventilation [ 21 – 23 ], while greater attention has been paid to this in paediatric and neonatal ventilation. The small V T of neonatal ventilation makes it crucial to consider the shunt compliance of the circuits when measuring volumes, either by excluding it by measuring flow after the circuit at the airways opening or by estimating and digitally compensating for it [ 24 , 25 ]. Also, specific design of the ventilation circuits improves the transmission of ventilation waveforms to the patient with possible consequences on the overall treatment [ 26 ].
Digital signal processing algorithms
Compared to previous generations of mechanical ventilators, the use of microprocessors allowed the development of sophisticated signal processing algorithms to compensate for intrinsic flaws in sensor technologies, consequently improving performance. As a drawback, these algorithms are mostly unknown to the users, who thus cannot understand or predict the behaviour of the ventilator in situations and conditions different from these considered during the development of the algorithms.
As volume measurements are obtained by digital integration of the flow signals, the signal processing also significantly contributes to volume accuracy by compensating for drift and leaks, especially during noninvasive ventilation, where leak estimation and compensation algorithms play a major role. Recent studies have shown that, even if compensation algorithms can markedly reduce measurement errors, overall performance are not yet ideal [ 16 , 27 ] and improvements are still needed, especially during variable leaks [ 28 ].
Maintenance
Even if each mechanical ventilator undergoes accurate calibration and testing before being set in operation, preserving performance over time is the responsibility of maintenance and servicing procedures, which have not always shown to be appropriate [ 29 ]. Even when the same model of mechanical ventilator is considered, wide variability in performance between devices has been observed ( figures 3 and 4 ), both in the high-end machines used in the ICU [ 17 ] and in simpler units for home ventilation [ 30 ], highlighting the urgency of improving lifetime quality control of such devices, especially for flow sensors.
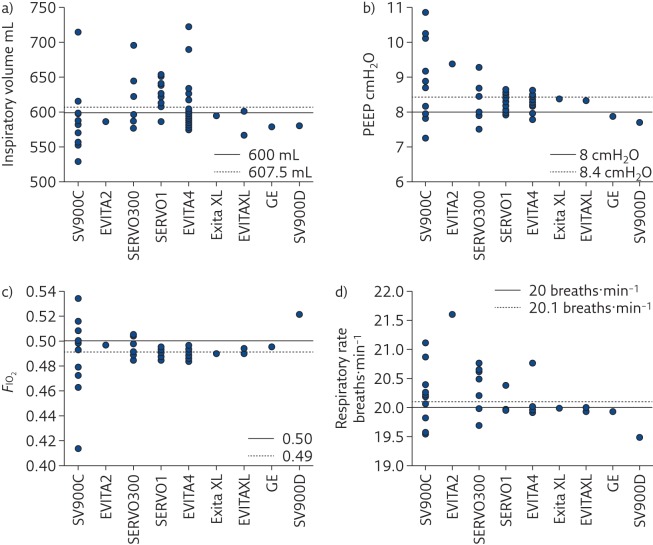
Measured values of a) inspiratory volume, b) PEEP, c) F IO 2 and d) respiratory rate delivered to a test lung by several devices in four intensive care units grouped by ventilator model. EVITA4: Draeger Evita 4 (25 machines); SERVOI: Siemens/Maquet Servo I (16 machines); SVC900C: Siemens SV900C (12 machines); SERVO300: Siemens/Maquet Servo 300 (seven machines); EVITAXL: Draeger Evita XL (three machines); SV900D: Siemens SV900D (one machine); EVITA2: Draeger Evita 2 (one machine) and ENGSTROM: GE Engstrom (one machine). Reproduced and modified from [ 17 ] with permission from the publisher.
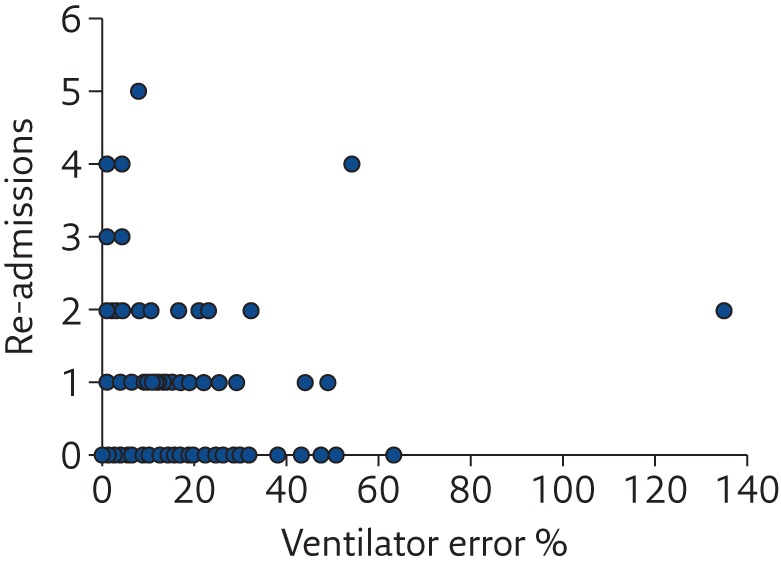
Relationship between the number of unplanned hospital re-admissions of home-ventilated patients in the previous year and the index of error of the home ventilator. Reproduced and modified from [ 30 ] with permission from the publisher.
Ventilation modes
Even if the concept of mechanical ventilation is apparently simple, there are virtually infinite possible patterns of interaction between the ventilator and the patient, namely the modes of ventilation. Originally, the classification of these modes was relatively simple, with “controlled ventilation modes” being used when the ventilator was imposing its action to the patient and “assisted ventilation modes” used when the ventilator was supporting the breathing of the patient. However, since the introduction of microprocessors, the number of modes of ventilation has grown exponentially. Moreover, in addition to the implicit complexity of this matter, many manufacturers have introduced different (and often trademarked) names for similar modes, mostly for pursuing marketing and branding strategies, making it even more difficult for the user to understand the principles and ideal use of each ventilation mode available on a given ventilator.
This is a relevant issue that probably deserves more attention in order to improve our ability to provide the best ventilation to our patients, whichever ventilator is used. Starting in 1992, Robert Chatburn developed a structured approach that lead to a well-defined and structured taxonomy to name ventilation modes depending on their essential features, making it possible to understand the features of a ventilation modality from its name [ 31 ].
Chatburn’s approach ( figure 5 ) is based on the definition of a mode of mechanical ventilation as the combination of three elements:
1) the ventilator breath control variable
2) the breath sequence
3) the targeting scheme
They used 10 maxims to progressively describe how to classify a given ventilation mode by understanding what it does ( table 1 ). Flow charts are also proposed to suggest a procedure for classifying a given ventilation mode by applying the maxims. Unfortunately, the introduction of such a structured taxonomy into clinical practice requires relevant and simultaneous efforts both in the educational programmes of medical schools and in the implementation of this naming approach in the ventilators by the manufacturers. This is unlikely to occur without a firm and combined effort from all medical societies of anaesthesia, intensive care and respiratory medicine, which is still lacking.
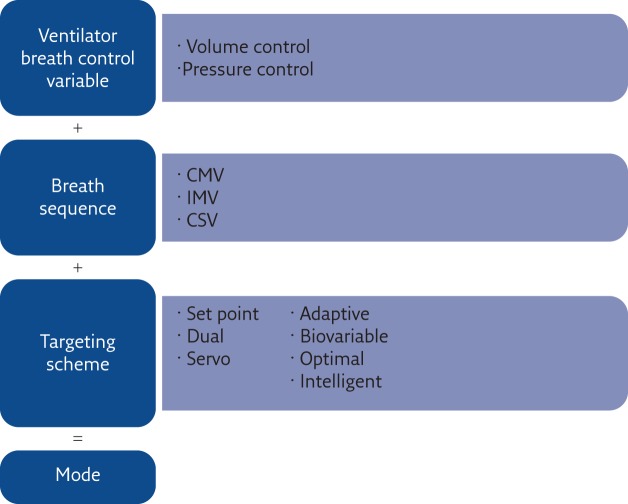
Construction of the ventilation mode taxonomy suggested by Chatburn. The name of a ventilation mode results from three elements. CMV: continuous mandatory ventilation; IMV: intermittent mandatory ventilation; CSV: continuous spontaneous ventilation. Reproduced and modified from [ 31 ] with permission from the publisher.
Chatburns’ maxims for understanding ventilator operation
CMV: continuous mandatory ventilation; IMV: intermittent mandatory ventilation; CSV: continuous spontaneous ventilation; VC: volume control; PC: pressure control. Reproduced and modified from [31] with permission from the publisher.
Since in-depth description of different ventilation modes is outside the goal of this review, the reader can find detailed information elsewhere [ 32 ]. As most of the recent innovation in mechanical ventilation is focused in developing new approaches for the implementation of a targeting scheme, the following paragraphs are focused on this topic, using Chatburn’s classification as reference ( table 2 ).
Classification of different targeting schemes
Reproduced and modified from [31] with permission from the publisher.
Targeting schemes 1 and 2: set-point and dual schemes
The set-point and dual schemes are both manual targeting schemes in which the operator directly adjusts the controlled output [ 33 ]. The majority of ventilation modalities is included in this category. These modes can be divided into synchronised or non-synchronised ventilation modes.
In synchronised modes, the advances of technologies resulted in improved trigger performance [ 34 – 36 ]. While in adults, the use of either the patient’s flow or pressure triggering results in non-clinically significant difference in performance [ 37 ], in neonatal ventilation, where V T is extremely small and respiratory rates high, flow triggering improves synchrony [ 38 ]. However, the patient’s triggering is still an open issue, especially in presence of leaks and during noninvasive ventilation [ 39 , 40 ].
Targeting scheme 3: servo targeting schemes
Servo targeting ventilation modes are characterised by the ventilator generating an output that follows a varying input in real time. Automatic tube compensation (ATC), neurally adjusted ventilator assist (NAVA), proportional assist ventilation (PAV) and PAV-plus are examples of this category. They allow high degree of synchrony with patients and support the work of breathing, assigning to the patient’s neural respiratory control the definition of the ventilation waveform.
ATC mathematically estimates the pressure drop due to the endotracheal tube (ETT) from the patient flow and continuously adjusts the pressure at the airway opening in order to maintain the pressure at the tip of the ETT at the set value, compensating (only) for the resistive workload added to the patient by the high resistance of the tube. NAVA measures the electrical activity of the diaphragm by transoesophageal electromyography and applies an airway opening pressure proportional to it to the patient. It allows better synchronisation with the patient, the delivery of physiological waveforms and it is robust in case of leaks and auto-PEEP [ 41 , 42 ]. PAV and PAV-plus support both the elastic and resistive work of the patient, and are based on the application of the equation of motion of the respiratory system, therefore requiring the knowledge of the patient’s respiratory resistance ( R dyn ) and compliance ( C dyn ). PAV-plus continuously monitors lung mechanics in order to adjust R dyn and C dyn to follow changes in patient’s conditions and resulting in better performance [ 33 ].
Targeting scheme 4: adaptive targeting
Adaptive targeting allows the ventilator to automatically set one ventilation variable to maintain another ventilation variable at a predefined value. In this way, the ventilator can cope with changes in patient conditions. Examples of ventilation modes using this scheme are: mandatory rate ventilation, in which the inspiratory pressure is modified to maintain the set respiratory frequency; adaptive flow/adaptive inspiratory time, in which the inspiratory time and inspiratory flow are automatically adjusted to maintain an inspiratory/expiratory ratio of 1/2; adaptive pressure control, in which inspiratory pressure is adapted to deliver a target V T regardless of any change in lung mechanics or patient effort; and mandatory minute ventilation, in which minute ventilation is maintained close to a target value by increasing/decreasing mandatory breaths or by an automatic adjustment of the inspiratory pressure. It is worthy to underline that these ventilation modes cannot be considered forms of automatic weaning [ 33 ].
Targeting scheme 5: biovariable targeting
Variable ventilation is a ventilation modality that provides a variable V T or peak pressure by adding random fluctuations to the set value in order to mimic the natural variability of the respiration. The rationale is based on the noise-enhanced amplification of a useful signal in a nonlinear system by stochastic resonance. Indeed, including appropriately designed noise in mechanical ventilators improves gas exchange [ 43 ]. Moreover, variability in the respiratory system has been found to be important for cell activity, as it positively modulates surfactant secretion [ 44 , 45 ]; it is also related to the maturation of breathing control in infants [ 46 , 47 ] and correlates with weaning success [ 48 – 50 ]. Animal studies have shown that variability improves ventilation efficiency and lung compliance in the preterm lung without increasing lung inflammation or lung injury [ 51 , 52 ].
Adjusting mechanical ventilation for patients’ condition and needs: optimal and intelligent targeting schemes
When, in 1998, Dreyfuss and Saumon demonstrated that the key ventilation variable responsible for the development of VILI is not the absolute pressure applied to the lung but the V T , it became clear that, in order to avoid VILI, the settings of ventilator parameters must be accurately tailored to the individual patient. However, even if the V T was physiologically corrected by adjusting it for body weight, many patients were injured anyway. A significant advancement in our understanding of VILI was provided by the use of computed tomography (CT) to study ARDS. It was thanks to CT scans that we learned that ARDS is not a homogeneous, but a highly heterogeneous, disease [ 53 , 54 ]. Three-dimensional lung images showed that in a diseased lung, there are nonaerated regions due to either a complete filling of the alveolar spaces with liquid and cells, and/or to the collapse of potentially recruitable pulmonary units (atelectasis) [ 55 ]. This has major consequences in tailoring mechanical ventilation: a V T estimated from the body size of a given patient might be excessive as the actual aerated lung can be much smaller than its anatomical dimensions (a concept called “baby lung”). The translation of this concept to clinical practice was demonstrated by the ARDSNet trial in 2000, in which a significant reduction of mortality was shown when a V T of 6 mL⋅kg −1 was used instead of 12 mL⋅kg −1 [ 56 ]. This and other studies underlined the importance of applying a lung-protective ventilation strategy in which the minimisation of side-effects of ventilation gains priority when applying this treatment. However, even if we now understand much more of the pathophysiology of ventilation, we are still not able to fully implement these concepts in the clinical practice. For example, we learned that ventilation parameters should be adjusted on the amount of the aerated lung of the patient and not simply on his/her body weight. However, the size of the ventilated lung changes from patient to patient and in the same patient with time. Moreover, as part of the nonaerated regions can be due to atelectasis, the application of different PEEP, ventilatory patterns and lung volume history can markedly impact the dimension of the baby lung by recruiting or de-recruiting atelectatic regions, thus making the baby lung size a highly dynamic variable and not a static one [ 57 , 58 ], thus requiring continuous adjustment of ventilator parameters. Therefore, PEEP should be optimised aiming to maximise the fraction of recruited lung [ 59 , 60 ], thus reducing inspiratory pressures as much as possible to minimise over-distention of lung tissues [ 56 ]. Unfortunately, the only clinically available validated technology for assessing lung volume recruitment is CT, which is obviously unsuitable for long-term bedside monitoring.
This highlights the importance of both a proper understanding of the pathophysiological mechanisms and quantification of related relevant variables in optimising ventilation. Similar reasoning applies to other specific conditions, such as the presence of intrinsic PEEP in obstructed patients and the controlled unloading of the respiratory muscles during weaning.
Even if these physiopathology concepts are now well understood, the key variables needed to tailor ventilation are not yet measurable at the bedside, leading to a significant gap between what we know about an ideal ventilation strategy and what can be applied in the clinic. For this reason, research efforts have been devoted in recent years to improving technologies for assessing physiological variables at the bedside and to incorporate into mechanical ventilators these enhanced monitoring capabilities for providing the clinicians with the information needed to properly tailor mechanical ventilation. Moreover, availability of measurements of specific physiological variables enables the implementation of automatic tailoring algorithms.
Monitoring physiological conditions
Pressure at the airway opening, ventilation volumes and F IO 2 are monitored by all modern mechanical ventilators. Beside oxygenation, which can be easily measured at bedside by pulse oximetry, distribution of lung ventilation and mechanics are among the most relevant variables for optimising ventilation. Therefore, recently developed noninvasive techniques for lung function monitoring are now becoming available in mechanical ventilators, allowing bedside monitoring of new relevant variable for optimising ventilation.
Monitoring distribution of ventilation: electrical impedance tomography
After decades of research, electrical imperdance tomography (EIT) is now commercially available to monitor the pattern of regional ventilation of the lungs during mechanical ventilation, and to show how it changes by modifying ventilation mode and parameters [ 61 ]. Recent studies showed that EIT can be an effective tool to titrate optimal PEEP [ 62 – 65 ].
Lung mechanics: forced oscillation technique
With the forced oscillation technique (FOT), a small-amplitude, high-frequency oscillatory pressure is applied to the airway opening, and the study of the relationship between the applied pressure and the resulting flow at different frequencies (the so-called respiratory impedance) allows the characterisation of specific features of the lung mechanical properties, even in presence of spontaneous breathing and without requiring oesophageal manometry [ 66 ]. In particular, recent studies have demonstrated potential usefulness of FOT for monitoring lung mechanics during mechanical ventilation [ 67 – 70 ], proved that it is sensitive and specific to changes in peripheral lung mechanics [ 71 – 73 ], and showed that FOT can be used as bedside tool for monitoring recruitment/derecruitment [ 74 ]. Specifically, monitoring lung mechanics by FOT during a decremental PEEP trial allows the identification of the open-lung PEEP, defined as the minimum PEEP level required to prevent lung derecruitment after a recruitment manoeuvre, with high sensitivity and specificity when compared with CT [ 75 , 76 ]. FOT may also be a useful tool for optimising PEEP in obstructed patients as it allows the monitoring of the presence of tidal expiratory flow limitation [ 71 , 72 ] and, in general, can be used to monitor the status and evolution of respiratory mechanics in the ventilated patient.
These are only examples of how cutting-edge technologies have resulted in new monitoring tools for optimising ventilation. Other physiological variables and measurement technologies have been studied for monitoring blood gases, lung mechanics, proinflammatory mediators, etc. [ 77 ].

Computational tools
The idea of automating mechanical ventilation was suggested in 1957 [ 78 ]. The rationale of “intelligent” ventilators is to improve patient management by analysing and integrating information coming from large number of sources, and guaranteeing continuous adjustment of the ventilation even when expert personnel are not constantly available, improving patients’ treatment and minimising clinical errors. Moreover, these tools can be also used for educational purposes.
However, ventilation management is a complex process involving the analysis of multiple parameters, subjective strategies and multiple goals (gas exchange, lung protection and weaning). Physiology, as well as the peculiarity of the pathology, must be taken into account, several clinical data must be gathered from many sources and the condition of the patient must be monitored continuously. Finally, priorities of goals, clinical preferences and local clinical protocols must be also considered.
Until now, different variables have been considered as input for optimising ventilation strategies. They include variables describing the general condition of the patients, haemodynamic variables, gas exchange, lung mechanics and the work of breathing [ 79 ]. When implementing these strategies in mechanical ventilators, there is the need to identify a trade-off between the complete description of the patient condition and the complexity of managing many variables, availability of the data, invasiveness of measurements and acquisition of data from different equipment.
The same can be said for the output variables: the simplest system controls only one variable. This is the case for algorithms that adjust F IO 2 to maintain target saturation. Ideally, on the basis of the condition of the patient, algorithms should be able to control not only a single variable but all the ventilator settings and even suggest specific manoeuvres or pharmacological treatments to the clinician.
Engineering science has developed several computational tools that start from a set of input (measured) variables and can determine the desired outputs (settings) [ 79 ]. They include mathematical models and classical controllers ( e.g. proportional, integrative and derivative controllers), rule-based expert systems ( e.g. systems that use coded clinical protocols), and other artificial intelligence techniques (fuzzy logic, neural networks and genetic algorithms) or hybrid systems (combinations of the aforementioned systems). All these engineering technologies can be used to model the cardiorespiratory system and/or the decisional process of the clinicians to automatically identify an optimal ventilation strategy.
Mathematical models have the advantage of describing pathophysiological processes. However, in order to be patient-specific, the identification of the parameters of the model is required. This is often difficult as these parameters are usually not measurable with noninvasive techniques and estimation errors, being unavoidable, must be considered. Classical control science controllers are commonly used to modify one or few ventilator parameters to maintain one or few physiological variables within a predefined range.
Rule-based expert systems are built using the available ventilation protocols and clinician expertise. They have the disadvantages of being related to protocols that may be subjective and specific for single centres.
Artificial intelligence techniques do not require modelling the system on a mechanistic basis. They are able to handle large number of variables and they can be trained on large existing data sets. As a limitation, their outputs are difficult to predict as they are determined by the complex interaction of many rules.
All these tools have been and are currently applied to implement both open-loop (decision support) systems and closed-loop systems. Closed-loop systems have the major advantage of allowing a continuous adaptation of ventilation to the patient without requiring the intervention of clinicians. However, as they apply changes to a patient’s therapy without involving, or at least requiring, the acknowledgment of a clinician, they open serious issues of safety and accountability [ 79 ] that will require, in some countries, compliance with current legislation.
Targeting scheme 6: optimal targeting schemes
These schemes allow automatic adjustment of ventilator settings to either minimise or maximise some overall performance characteristic. Different optimal targeting schemes have been proposed in the literature [ 79 ]. They include:
schemes involving a simple controller used for the automatic adjustment of F IO 2 to maintain patient saturation within an optimal range, or to adjust inspiratory pressure or minute ventilation on the basis of carbon dioxide exchange
more complex procedures including mathematical models and explicit objective functions for optimising more than one variable ( e.g. optimising respiratory rate, V T , inspiratory/expiratory time and PEEP for minimising the elastance of the respiratory system and regulating end-tidal carbon dioxide)
A ventilation modality available in commercial mechanical ventilators that follows this scheme is adaptive support ventilation (ASV). ASV is designed to minimise the work of breathing. Its inputs are body weight, F IO 2 , PEEP and %MV to be supported by the machine, and the target is to automatically adjust respiratory rate and V T , considering resistance, compliance and auto-PEEP. Some expert rules are used to maintain frequency and V T in safe ranges and reduce the risk of auto-PEEP. Because of these rules, ASV can be better classified as a form of hybrid scheme that combines mathematical modelling and artificial intelligence. Studies have shown that ASV can select specific V T and respiratory rate settings [ 80 ] to achieve the same arterial partial pressure of carbon dioxide as the clinicians do and allowing weaning automation [ 81 ].
The new modality Intellivent-ASV adds to the ASV controller (ventilation controller) an oxygenation controller that adjusts PEEP and F IO 2 , depending on the ARDS network tables to maintain oxygen saturation measured by pulse oximetry ( S pO 2 ) within a pre-set range. Moreover, it includes a test for probing weaning readiness of patients [ 82 ].
Targeting scheme 7: intelligent targeting schemes
Several systems were described in literature for setting ventilation parameters based on some form of artificial intelligence. Examples of this targeting scheme are a fuzzy-logic system used for F IO 2 control, or for optimising respiratory rate and V T on the basis of lung mechanics, S pO 2 , carbon dioxide, body temperature, end-tidal carbon dioxide, and the patient’s body weight and height or to implement the “open-lung approach”; and artificial neural networks, genetic algorithms, fuzzy-logic systems and their combination for the support of ventilation management specific to some common lung pathologies [ 79 ].
Studies applying rule-based expert systems include those by L aubscher et al. [ 83 ], which was included in the development of ASV, and D ojat et al. [ 84 ], which was integrated in a commercial ventilator under the name of Smartcare. Smartcare is a rule-based expert system for automatic control of pressure support ventilation. It processes respiratory rate, end-tidal carbon dioxide, pressure support and V T . It aims to maintain the patient in a “comfort zone” defined by a combination of V T , respiratory frequency and end-tidal carbon dioxide, and to progressively decrease the inspiratory pressure. Studies assessing the impact of using this ventilation mode found that it has better or similar performance to weaning managed by experienced personnel [ 85 – 89 ]. However, it is not well suited for patients with low spontaneous breathing activity or during worsening of airway obstruction [ 82 ].
Educational perspective
This review has mainly focused on describing how the combination of physiology, medicine and engineering concepts and tools are being used to improve mechanical ventilation. To go further, two kinds of advancements are required. The first is the development of cost-effective, accurate, miniaturised and reliable sensors to measure patient variables ( e.g. pressure, flow and oxygenation) and actuators to build machines reliably providing the desired pressure/flow to patients ( e.g. blowers and valves). The second is the application of mathematical models, control systems and artificial intelligence to tailor the action of the mechanical ventilator to each patient’s needs. While progress in sensors and actuators is mostly a technological issue (relatively independent of respiratory pathophysiology), application of control/intelligence tools to the specific case of mechanical ventilation is something that goes beyond the pure discipline of engineering, since it requires a deep knowledge of the pathophysiology of the respiratory system. This is a challenging task that must be carried out by a truly interdisciplinary approach, joining the knowledge and expertise of bioengineers and physicians. Although this kind of approach has already been followed “informally” since the beginning of mechanical ventilation, interdisciplinary efforts are more and more necessary as the complexity of modern mechanical ventilators increases as these machines are among the most complex medical devices in terms of integrating and controlling a variety of input and output variables.
It goes without saying that bioengineers focused on designing and building mechanical ventilators must be deeply educated on respiratory pathophysiology. However, it is nowadays not so obvious that physicians dealing with mechanical ventilation have been sufficiently educated on the technological aspects of these complex medical devices. Physicians attending patients under acute or chronic mechanical ventilation are faced with considerable difficulties to properly understand how these devices work. As mentioned before in this review, a major difficulty is the intrinsic complexity of modern control/intelligence algorithms implemented in current devices. This difficulty is increased by the lack of standardisation and the fact that, for marketing and commercial strategies, companies building ventilators tend to add new definitions of ventilation modes and variants without disclosing the algorithms with sufficient detail for the user to really understand how the machine is working. The result is that in most cases the physician is using a sort of “black box” as therapeutic device. Accordingly, it seems necessary that, when being specifically trained to use mechanical ventilation, physicians should be also provided with a sufficient education on the technological issues involved in these modern medical devices. In that way, they will be in optimal professional conditions to select the best therapeutic option for each individual patient.
Conclusions
Patients’ needs are changing with time because of the evolution of both pathology severity and treatments. Therefore, an optimal ventilation strategy is an adaptive process aiming to treat the acute condition and either support the gradual weaning from the ventilator or adjust to the natural fluctuations of chronic disorders. Such a tailoring could improve outcomes and comfort, reduce adverse events, and avoid prolonging ventilation, with its associated risks and costs. To reach these goals, advancement of our understanding in pathophysiology, medicine and engineering must be combined with an interdisciplinary approach to fully address the complexity of mechanical ventilation for further improving patients’ treatment.
Educational questions
- c) Only at the beginning of their life cycle
- a) Yes, all ventilators use only standardised names
- b) No, there might be different names for the same mode as manufacturers are not obliged to use a standardised taxonomy
- c) Only intensive care unit ventilators are required to use a standardised taxonomy
- b) Only in modern mechanical ventilators
- c) No, in modern mechanical ventilators the use of different data processing algorithms can lead to very different overall performance of the same sensor technology
- d) Only for flow sensors
- a) The optimal targeting scheme identifies the best ventilation setting while the intelligent scheme, the best ventilation mode
- b) The optimal targeting scheme adjust ventilation parameters in order to maximise or minimise a comprehensive physiological feature such as work of breathing, while the intelligent scheme uses artificial intelligence tools to optimise the ventilation settings on the basis of coded knowledge
- c) The two terms refer to the same approach
- a) Similar for all variables
- b) Higher for volumes compared to pressure, F IO 2 and time
- c) Lower for pressure compared to volume
- d) Higher for pressure compared to F IO 2 and time
Suggested answers
Disclosures.
R. Dellaca EDU-0078-2017_Dellaca (1.2MB, pdf)
C. Veneroni EDU-0078-2017_Veneroni (1.2MB, pdf)
Conflict of interest Disclosures can be found alongside this article at breathe.ersjournals.com
- 1. Vesalius A. De humani corporis fabrica. 1543.
- 2. Dalziel J. On sleep and apparatus for promoting artificial respiration. Br Assoc Adv Sci 1838; 2: 127–134. [ Google Scholar ]
- 3. Lassen HCA. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953; 1: 37–41. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Dixon WE, Brodie TG. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J Physiol 1903; 29: 97–173. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Rohrer F. Der Zusammenhang der Atemkräfte und ihre Abhängigkeit vom Dehnungszustand der Atmungsorgane. Pflügers Arch Eur J Physiol 1916; 165: 419–444. [ Google Scholar ]
- 6. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970; 28: 596–608. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Rahn H, Otis AB, Chadwick LE, et al. . The pressure-volume diagram of the thorax and lung. Am J Physiol 1946; 146: 167–178. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Ashbaugh DG, Bigelow DB, Petty TL, et al. . Acute respiratory distress in adults. Lancet 1967; 2: 319–323. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Downs JB, Klein EF, Desautels D, et al. . Intermittent mandatory ventilation: a new approach to weaning patients from mechanical ventilators. Chest 1973; 64: 331–335. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Fry DL, Hyatt RE. Pulmonary mechanics. A unified analysis of the relationship between pressure, volume and gasflow in the lungs of normal and diseased human subjects. Am J Med 1960; 29: 672–689. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Hyatt RE, Schilder DP, Fry DL. Relationship between maximum expiratory flow and degree of lung inflation. J Appl Physiol 1958; 13: 331–336. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Campbell E. The Respiratory Muscles and the Mechanics of Breathing. London, Lloyd-Luke, 1958. [ Google Scholar ]
- 13. Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol 1967; 22: 407–422. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 1974; 110: 556–565. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Thille AW, Lyazidi A, Richard J-CM, et al. . A bench study of intensive-care-unit ventilators : New versus old and turbine-based versus compressed gas-based ventilators ventilators. Intensive Care Med 2009; 35: 1368–1376. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Garnier M, Quesnel C, Fulgencio J-P, et al. . Multifaceted bench comparative evaluation of latest intensive care unit ventilators. Thompson JP, editor. Br J Anaesth 2015; 115: 89–98. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Govoni L, Dellaca RL, Penuelas O, et al. . Actual performance of mechanical ventilators in ICU: A multicentric quality control study. Med Devices Evid Res 2012; 5: 111–119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Leipälä JA, Iwasaki S, Milner A, et al. . Accuracy of the volume and pressure displays of high frequency oscillators. Arch Dis Child Fetal Neonatal Ed 2004; 89: F174–F176. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Schena E, Massaroni C, Saccomandi P, et al. . Flow measurement in mechanical ventilation: a review. Med Eng Phys 2015; 37: 257–264. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Silvestri S, Schena E. Micromachined flow sensors in biomedical applications. 2012; 3: 225–243. [ Google Scholar ]
- 21. Hess D, McCurdy S, Simmons M. Compression volume in adult ventilator circuits: a comparison of five disposable circuits and a nondisposable circuit. Respir Care 1991; 36: 1113–1118. [ PubMed ] [ Google Scholar ]
- 22. Disposable adult breathing circuits for use with critical care ventilators. Health Devices 1994; 23: 104–129. [ PubMed ] [ Google Scholar ]
- 23. Wang JS, Hung WT, Lin CY. Leakage of disposable breathing circuits. J Clin Anesth 1992; 4: 111–115. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Castle RA, Dunne CJ, Mok Q, et al. . Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med 2002; 30: 2566–2574. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Cannon ML, Cornell J, Tripp-Hamel DS, et al. . Tidal volumes for ventilated infants should be determined with a pneumotachometer placed at the endotracheal tube. Am J Respir Crit Care Med 2000; 162: 2109–2112. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Wheeler KI, Moore GP, Morley CJ, et al. . Comparison of two ventilator circuits for Drager Babylog high-frequency ventilation. J Paediatr Child Health 2011; 47: 211–216. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Itagaki T, Bennett DJ, Chenelle CT, et al. . Performance of leak compensation in all-age ICU ventilators during volume-targeted neonatal ventilation: a lung model study. Respir Care 2017; 62: 10–21. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Lujan M, Sogo A, Grimau C, et al. . Influence of dynamic leaks in volume-targeted pressure support noninvasive ventilation: a bench study. Respir Care 2015; 60: 191–200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Beydon L, Liu N, Hassapopoulos J, et al. . Test of 20 similar intensive care ventilators in daily use conditions--evaluation of accuracy and performances. Intensive Care Med 1992; 18: 32–37. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Farré R, Navajas D, Prats E, et al. . Performance of mechanical ventilators at the patient’s home: a multicentre quality control study. Thorax 2006; 61: 400–404. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Chatburn RL, El-Khatib M, Mireles-Cabodevila E. A taxonomy for mechanical ventilation: 10 fundamental maxims. Respir Care 2014; 59: 1747–1763. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Cairo JM. Mosby’s Respiratory Care Equipment. Amsterdam, Elsevier Health Sciences, 2013. [ Google Scholar ]
- 33. Chatburn RL, Mireles-Cabodevila E. Closed-loop control of mechanical ventilation: description and classification of targeting schemes. Respir Care 2011; 56: 85–102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Richard J-C, Carlucci A, Breton L, et al. . Bench testing of pressure support ventilation with three different generations of ventilators. Intensive Care Med 2002; 28: 1049–1057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Thille AW, Lyazidi A, Richard J-CM, et al. . A bench study of intensive-care-unit ventilators: new versus old and turbine-based versus compressed gas-based ventilators. Intensive Care Med 2009; 35: 1368–1376. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Takeuchi M, Williams P, Hess D, et al. . Continuous positive airway pressure in new-generation mechanical ventilators: a lung model study. Anesthesiology 2002; 96: 162–172. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Kondili E. Patient-ventilator interaction. Br J Anaesth 2003; 91: 106–119. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Keszler M. State of the art in conventional mechanical ventilation. J Perinatol 2009; 29: 262–275. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Oto J, Chenelle CT, Marchese AD, et al. . A comparison of leak compensation in acute care ventilators during noninvasive and invasive ventilation: a lung model study. Respir Care 2013; 58: 2027–2037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Stell IANM, Paul G, Lee KC, et al. . Noninvasive ventilator triggering in chronic a test lung comparison. Am J Respir Crit Care Med 2001; 164: 2092–2097. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Lellouche F, Brochard L. Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, SmartCare). Best Pract Res Clin Anaesthesiol 2009; 23: 81–93. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Branson RD, Johannigman JA. Innovations in mechanical ventilation. Respir Care 2009; 54: 933–947. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Suki B, Alencar AM, Sujeer MK, et al. . Life-support system benefits from noise. Nature 1998; 393: 127–128. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Arold SP, Suki B, Alencar AM, et al. . Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol 2003; 285: L370–L375. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 2009; 296: L574–L581. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Frey U, Silverman M, Barabási AL, et al. . Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol 1998; 85: 789–797. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Engoren M, Courtney SE, Habib RH. Effect of weight and age on respiratory complexity in premature neonates. J Appl Physiol 2009; 106: 766–773. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Wysocki M, Cracco C, Teixeira A, et al. . Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med 2006; 34: 2076–2083. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Kaczmarek J, Kamlin COF, Morley CJ, et al. . Variability of respiratory parameters and extubation readiness in ventilated neonates. Arch Dis Child Fetal Neonatal Ed 2013; 98: F70–F73. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Engoren M. Approximate entropy of respiratory rate and tidal volume during weaning from mechanical ventilation. Crit Care Med 1998; 26: 1817–1823. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Berry CA, Suki B, Polglase GR, et al. . Variable ventilation enhances ventilation without exacerbating injury in preterm lambs with respiratory distress syndrome. Pediatr Res 2012; 72: 384–392. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Pillow JJ, Musk GC, McLean CM, et al. . Variable ventilation improves ventilation and lung compliance in preterm lambs. Intensive Care Med 2011; 37: 1352–1359. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Gattinoni L, Mascheroni D, Torresin A, et al. . Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive Care Med 1986; 12: 137–142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Maunder RJ, Shuman WP, McHugh JW, et al. . Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA 1986; 255: 2463–2465. [ PubMed ] [ Google Scholar ]
- 55. Remy-Jardin M, Remy J, Giraud F, et al. . Computed tomography assessment of ground-glass opacity: semiology and significance. J Thorac Imaging 1993; 8: 249–264. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Acute Respiratory Distress Syndrome Network,Brower RG, Matthay MA, et al. . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Crotti S, Mascheroni D, Caironi P, et al. . Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 2001; 164: 131–140. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Pelosi P, Goldner M, McKibben A, et al. . Recruitment and derecruitment during acute respiratory failure. Am J Respir Crit Care Med 2001; 164: 122–130. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Brower RG, Lanken PN, MacIntyre N, et al. . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351: 327–336. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Gong B, Krueger-Ziolek S, Moeller K, et al. . Electrical impedance tomography : functional lung imaging on its way to clinical practice? Expert Rev Respir Med 2015; 9: 721–737. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Wolf GK, Gómez-Laberge C, Rettig JS, et al. . Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med 2013; 41: 1296–1304. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Liu S, Tan L, Möller K, et al. . Identification of regional overdistension, recruitment and cyclic alveolar collapse with electrical impedance tomography in an experimental ARDS model. Crit Care 2016; 20: 119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Muders T, Luepschen H, Zinserling J, et al. . Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit Care Med 2012; 40: 903–911. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Costa EL V, Borges JB, Melo A, et al. . Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009; 35: 1132–1137. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Kaczka DW, Dellacà RL. Oscillation mechanics of the respiratory system: applications to lung disease. Crit Rev Biomed Eng 2011; 39: 337–359. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Farre R, Ferrer M, Rotger M, et al. . Servocontrolled generator to measure respiratory impedance from 0.25 to 26 Hz in ventilated patients at different PEEP levels. Eur Respir J 1995; 8: 1222–1227. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Bellardine CL, Ingenito EP, Hoffman A, et al. . Heterogeneous airway versus tissue mechanics and their relation to gas exchange function during mechanical ventilation. Ann Biomed Eng 2005; 33: 626–641. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Kaczka DW, Hager DN, Hawley ML, et al. . Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology 2005; 103: 306–317. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Kaczka DW, Brown RH, Mitzner W. Assessment of heterogeneous airway constriction in dogs: a structure-function analysis. J Appl Physiol 2008; 106: 520–530. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Dellacà RL, Rotger M, Aliverti A, et al. . Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J 2006; 27: 983–991. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Dellaca RL, Santus P, Aliverti A, et al. . Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 2004; 24: 332–333. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Johnson MK, Birch M, Carter R, et al. . Use of reactance to estimate transpulmonary resistance. Eur Respir J 2005; 25: 1061–1069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Dellaca RL, Andersson Olerud M, Zannin E, et al. . Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med 2009; 35: 2164–2172. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Dellacà RL, Zannin E, Kostic P, et al. . Optimisation of positive end-expiratory pressure by forced oscillation technique in a lavage model of acute lung injury. Intensive Care Med 2011; 37: 1021–1030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Kostic P, Zannin E, Andersson Olerud M, et al. . Positive end-expiratory pressure optimization with forced oscillation technique reduces ventilator induced lung injury: a controlled experimental study in pigs with saline lavage lung injury. Crit Care 2011; 15: R126. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Nieman GF, Satalin J, Andrews P, et al. . Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensive Care Med Exp 2017; 5: 8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Saxton GA, Myers G. A servomechanism for automatic regulation of pulmonary ventilation. J Appl Physiol 1957; 11: 326–328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Tzavaras A, Weller PR. Classical approaches and intelligent systems in ventilation management: a survey. Crit Rev Biomed Eng 2010; 38: 157–188. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Arnal J, Nafati C, Wysocki M, et al. . Utilization of adaptive support ventilation (ASV) in a polyvalent intensive care unit. Intensive Care Med 2004; 30: S84. [ Google Scholar ]
- 81. Dongelmans DA, Veelo DP, Paulus F, et al. . Weaning automation with adaptive support ventilation: a randomized controlled trial in cardiothoracic surgery patients. Anesth Analg 2009; 108: 565–571. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Alam M, Jones G, Kahl W, et al. . Modeling the weaning of intensive care unit patients from mechanical ventilation: A review. Crit Rev Biomed Eng 2014; 42: 25–61. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Laubscher TP, Heinrichs W, Weiler N, et al. . An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41: 51–59. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Dojat M, Pachet F, Guessoum Z, et al. . NéoGanesh: a working system for the automated control of assisted ventilation in ICUs. Artif Intell Med 1997; 11: 97–117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Dojat M, Harf A, Touchard D, et al. . Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med 2000; 161: 1161–1166. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Lellouche F, Mancebo J, Jolliet P, et al. . A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med 2006; 174: 894–900. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Rose L, Presneill JJ, Johnston L, et al. . A randomised, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare TM /PS. Intensive Care Med 2008; 34: 1788–1795. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Schädler D, Engel C, Elke G, et al. . Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med 2012; 185: 637–644. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Burns KEA, Meade MO, Lessard MR, et al. . Wean Earlier and Automatically with New Technology (the WEAN Study). A multicenter, pilot randomized controlled trial. Am J Respir Crit Care Med 2013; 187: 1203–1211. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (499.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Mechanical Ventilator Support and Management Essay (Critical Writing)
Introduction, modes of mechanical ventilator support, strategies for managing patient responses to mechanical ventilator support, assessments and causes of patient-ventilator dyssynchrony.
The article emphasizes the need for nurses to embrace evidence-based knowledge of how the various modes of ventilators operate, on strategies for managing patient responses to mechanical ventilator support, and causes of patient-ventilator dyssynchrony. This will enable them to provide quality healthcare to the patients. Timely identification of potential threats to health by nurses goes a long way in containing respiratory challenges. In their article, Grossbach, Chlan, and Tracy (2011) offer a general discussion of the areas that nurses need to be highly informed about. The paper critically analyzes the content of the article by either supporting or dismissing claims made by the authors using reliable and valid evidence.
Grossbach et al. note that critically ill adults who are admitted in the intensive care unit (ICU) are the most common group of patients that would always require mechanical ventilator support (2005). This is echoed by the findings of a study to establish the characteristics and results of cancer patients in need of mechanical ventilator support for more than 24 hours (Soares, Salluh, Spector, & Rocco, 2005). They found that acuteness of the affected organ, status of cancer, and aging were some of the main factors affecting mortality in the ICU. The ventilator support is also used routinely in other settings where long-term and sub-acute care is provided. The use of this crucial information helps in enhancing the chances of survival of patients admitted in the intensive care unit. When using the mechanical ventilator support, the nurse is usually in charge of every detail and should be able to address all challenges that may arise.
Although the parameters of ventilators may differ with the manufacturing company, there are those which are found on all ventilators. These include the percentage of oxygen, tidal volume or minute ventilation, the rate of respiration, flow/inspiration rate, and alarm settings scale. Tehrani and Roum (2008) discuss a new computerized system for mechanical ventilation. Instead of a rule-based system, Flex is designed to work with the status of the patient being treated and hence can be used in several modes of ventilation. It can also regulate weaning to fit the conditions of the patient. Advanced types of ventilators incorporate newer settings and nurses should always be updated on ventilator operation for quality healthcare.
How the mechanical ventilator provides inspiratory support determines the mode of ventilation under use. Usually, a clinician relies on past experiences in choosing the mode of ventilation. The institution also has its preference when choosing the mode of ventilation. In their peer reviewed article, however, Branson and Johannigman (2004) observe that there is no evidence to show that new modes of ventilator support are superior to the existing ones. Just the same way that Flex functions, the authors of the article note that different modes operate differently especially in volume or pressure regulation. According to Burns (2009), the mode of ventilation to be used depends greatly on the ability of the patient to respond positively to it. The article captures other modes of mechanical ventilator support.
Despite the availability of different modes of mechanical ventilator support, some patients have been found to encounter major problems when using these services. Usually, the patient who is incompatible with a given ventilator support will appear distressed and struggle to breath. It is important to understand why this situation may arise. Since the ventilation is mechanical or computerized, it may in some cases be “out of synch” with the natural breathing rhythm. Simonds (2006) agree that almost all the mechanical ventilators are not free from malfunction. Worse still, those patients receiving home-based ventilator care are at greater risk due to higher chances of interruption and less timely response. The other risk factor for patient-ventilator problems is lack of skill in managing the operation of the mechanical ventilators especially among the homecare providers. Any delay to assess the cause of alarm will have dire consequences.
Sometimes, it gets even more serious when the patient is sedated or is unconscious because the alarm will not ring. This will only cause more ventilation problems. Barely half of the hospitals which encourage home based ventilation do follow up visits to determine the progress of the patient (Simonds, 2006). Through the entire process of mechanical ventilator support, the patient is the sole center of attention. This is aimed at ensuring that optimal outcomes of treatment are realized. The authors of the article discuss other factors that can affect the pressure and volume delivery targets of a mechanical ventilator as well as dyspnea.
Dyssynchrony occurs when there is a mismatch between the patient’s breath and the mechanical ventilator-assisted breaths. This particular phenomenon is one of the most serious factors affecting the outcome of treatment and may warrant prolonged stay in hospital or ventilator-assisted respiration. One of the most common causes of this situation is the failure of initial inspiration by the patient to trigger or match with the breath from the ventilator. It has been established that lack of sufficient breath leads to several symptoms like restlessness, anxiety, hypertension, and nasal flaring. Mechanical problems in the ventilator may also cause dyssynchrony. Other important causes of this problem are pointed out in the article; autotrigerring, insufficient inspiratory flow delivery, double-triggering, and exhalation phase dyssynchrony.
The paper has captured very critical areas discussed by Grossbach et al. (2011) on the overview of mechanical ventilator support and management of patient and ventilator-related responses. Nurses have to be thoroughly trained on how the various ventilator modes function as well as their limitations. They should also be aware of the causes of respiratory dyssynchrony with the mechanical ventilator support. Healthcare providers, therefore, should share their experiences with the use of ventilators to enhance the quality of care provided to patients and avoid any foreseeable problems when using mechanical ventilation support.
Branson, R. D., & Johannigman, J. A. (2004). What is the evidence base for the newer ventilation modes? Respiratory Care, 49 (7), 742–760.
Burns, M. S. (2009). Understanding mechanical ventilation support and weaning. Critical Care Nurse, 27 (5), 469–492.
Grossbach, I., Chlan, L., & Tracy, F. M. (2011). Overview of mechanical ventilator support and management of patient and ventilator-related responses. Critical Care Nurse, 31 (3), 30-44
Simonds, A. K. (2006). Risk management of the home ventilator dependent patient. THORAX, 61 (5), 369-371
Soares, M., Salluh, J. F., Spector, N., & Rocco, J. (2005). Characteristics and outcomes of cancer patients requiring mechanical ventilator support for > 24 hours. Critical Care Nurse, 33 (3), 520-526
Tehrani, F. T., & Roum, J. H. (2008). Flex: a new computerized system for mechanical ventilation. Journal of Clinical Monitoring and Computing, 22 (2), 121-130
- Advantages and Disadvantages of Electronic Medical Records
- Physical Hazards of Noise and Protection Measures
- Respiratory Failure in Critical Care Practice
- Evidence-Based Practice and Applied Nursing
- Intervention to Prevent Ventilator Associated Pneumonia
- Genetic Mapping in the United States
- Stereotypical Health Care Beliefs & Practices Description
- The Case of High Blood Pressure
- Diffusion of Innovations Theory
- Historical Role, Function, and Purpose of the Nightingale Pledge
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, May 18). Mechanical Ventilator Support and Management. https://ivypanda.com/essays/mechanical-ventilator-support-and-management/
"Mechanical Ventilator Support and Management." IvyPanda , 18 May 2022, ivypanda.com/essays/mechanical-ventilator-support-and-management/.
IvyPanda . (2022) 'Mechanical Ventilator Support and Management'. 18 May.
IvyPanda . 2022. "Mechanical Ventilator Support and Management." May 18, 2022. https://ivypanda.com/essays/mechanical-ventilator-support-and-management/.
1. IvyPanda . "Mechanical Ventilator Support and Management." May 18, 2022. https://ivypanda.com/essays/mechanical-ventilator-support-and-management/.
Bibliography
IvyPanda . "Mechanical Ventilator Support and Management." May 18, 2022. https://ivypanda.com/essays/mechanical-ventilator-support-and-management/.
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Mechanical ventilation.
Sean M. Hickey ; Abdulghani Sankari ; Al O. Giwa .
Affiliations
Last Update: March 30, 2024 .
- Continuing Education Activity
Mechanical ventilation is a critical intervention to sustain life in acute or emergent settings, particularly in patients with compromised airways, impaired ventilation, or hypoxemic respiratory failure. This procedure involves applying positive pressure breaths and relies on the airway system's compliance and resistance. Clinicians in critical care units must understand how mechanical ventilation affects patient physiology and response to various disease states, emphasizing the need for a deep understanding of safe application principles. A solid understanding of human physiology and airway mechanics principles is crucial for clinicians in treating intubated patients, forming the foundation for safe and effective ventilation strategies. This knowledge is essential for recognizing key indications for invasive mechanical ventilation, which include airway compromise, protection in obtunded patients or those with dynamic airways, airway obstruction, hypoventilation, and hypoxemia due to various pulmonary and systemic conditions. These principles also guide the selection of common ventilation modes, initial settings, and supportive care for intubated patients. This activity focuses on treating intubated patients during the initial hours of mechanical ventilation care, enabling participating clinicians to collaborate effectively with intensivists, emergency clinicians, or anesthesiologists. This activity provides interprofessional healthcare providers with fundamental knowledge regarding mechanical ventilation and ventilator strategies, ensuring preparedness for seamless collaboration with relevant healthcare providers to optimize patient outcomes.
- Identify key indications for invasive mechanical ventilation in patients with compromised airways, impaired ventilation, or hypoxemic respiratory failure.
- Implement safe and effective mechanical ventilation strategies tailored to individual patient needs and responses.
- Select appropriate ventilator modes, tidal volumes, respiratory rates, and positive end-expiratory pressure levels according to the patient's condition and therapy goals.
- Collaborate with interprofessional healthcare team members, including respiratory therapists and intensivists, to optimize ventilation strategies and patient outcomes.
- Introduction
Mechanical ventilation is a critical intervention to sustain life in acute or emergent settings, particularly in patients with compromised airways, impaired ventilation, or hypoxemic respiratory failure. This procedure involves applying positive pressure breaths and relies on the airway system's compliance and resistance. Clinicians in critical care units must understand how mechanical ventilation affects patient physiology and response to various disease states, emphasizing the need for a deep understanding of safe application principles. A solid understanding of human physiology and airway mechanics principles is crucial for clinicians in treating intubated patients, forming the foundation for safe and effective ventilation strategies. This knowledge is essential for recognizing key indications for invasive mechanical ventilation to select the common and appropriate ventilation modes, initial settings, and supportive care for intubated patients, which are reviewed below. Noninvasive ventilation is addressed separately. [1]
Indications for Invasive Mechanical Ventilation
The primary indications for invasive mechanical ventilation are categorized as follows:
- Patients who are obtunded or with dynamic airways need airway protection, such as those from trauma or oropharyngeal infection.
- Patients with airway obstruction can experience either proximal (such as angioedema) or distal issues (such as asthmatic bronchospasm or acute exacerbation of chronic obstructive pulmonary disease or COPD).
- Impaired central drive (such as drug overdose)
- Respiratory muscle weakness (such as muscular dystrophy and myositis)
- Peripheral nervous system defects (such as Guillain-Barré syndrome or myasthenic crisis)
- Restrictive ventilatory defects (such as chest wall trauma or disease or massive pneumothorax or effusion)
- Alveolar filling defects (such as pneumonia, acute respiratory distress syndrome (ARDS), or pulmonary edema)
- Pulmonary vascular defects leading to ventilation-perfusion mismatch (such as massive pulmonary embolism or air emboli)
- Diffusion defects (such as advanced pulmonary fibrosis)
- Increased ventilatory demand can result from conditions such as severe sepsis, shock, or severe metabolic acidosis. [2] [3] [4] [5] [6]
Mechanical ventilation operates by applying a positive pressure breath, relying on the compliance and resistance of the airway system. During spontaneous inspiration, the lung expands as transpulmonary pressure primarily stems from a negative pleural pressure generated by the inspiratory muscles. In contrast, controlled mechanical ventilation utilizes positive airway pressure to drive gas into the lungs, creating a positive-pressure environment. [7] Tidal volume (V T ) represents the air exchanged during each respiratory cycle. [8] Physiologically, V T is influenced by the individual's height and gender, typically between 8 and 10 mL/kg of ideal body weight. [2] Mechanical ventilation can be administered through various modes, including mandatory or assisted modes. In the assisted mode, the patient's inspiratory effort triggers the mechanical ventilation to deliver the breath. At the same time, the pressure is the product of negative pleural pressure and positive alveolar pressure.
The most common modes of mechanical ventilation include:
- Volume-limited assist control (VAC) ventilation
- Pressure-limited assist control (PAC) ventilation
- Synchronized intermittent mandatory ventilation (SIMV) with pressure support ventilation (PSV)
PSV is usually not used as a primary mode; instead, it is commonly used during the weaning process from mechanical ventilation. Other types of mechanical ventilation modes include controlled mechanical ventilation, which can be volume-limited or pressure-limited, as well as intermittent mandatory ventilation. Additionally, airway pressure release ventilation (APRV) or bilevel mechanical ventilation are less frequently used as initial settings. [9] Breath delivery in mechanical ventilation can generally be categorized as volume-limited or pressure-limited. Variations in tidal volume (V T ) and airway pressure occur depending on respiratory compliance, airway resistance, and the specific mode of mechanical ventilation used. For instance, VT is set to a fixed amount in VAC mode, with the static airway pressure (or plateau pressure at end inspiration) influenced by lung compliance. Conversely, in PAC mode, the driving pressure is set and fixed, resulting in variable V T from breath to breath, which depends on lung compliance (ie, higher lung compliance leads to higher V T , and lower lung compliance leads to lower V T ).
Mechanical ventilation comprises four stages: the trigger phase, the inspiratory phase, the cycling phase, and the expiratory phase. The trigger phase initiates inhalation, either prompted by the patient's effort or predefined parameters set by the mechanical ventilator. The inspiratory phase involves the intake of air into the patient's lungs. Following inspiration, the cycling phase denotes the cessation of inhalation but precedes the onset of exhalation. Lastly, the expiratory phase signifies the passive exhalation of air from the patient's lungs.
Setting Mechanical Ventilation
The mechanical ventilation modes mentioned above are used for initiation. The selection of mechanical ventilation mode should be personalized to ensure safety by optimizing ventilation-perfusion matching and the pressure-volume relationship of the lungs. [10] In addition, patient-ventilator synchrony and comfort are important factors for the mode selection.
VAC mode: When VAC mode is chosen, the following parameters have to be set on the ventilator:
- Tidal volume (V T ): The tidal volume is usually determined based on ideal or predicted body weight (PBW) rather than actual weight. In conditions such as ARDS that require a protective lung strategy, the V T is set at a low range of 4 to 8 mL/kg PBW.
- Respiratory rate (RR): The respiratory rate is typically between 12 and 16 breaths per minute. A higher respiratory rate (up to 35 breaths per minute) may be selected to achieve sufficient minute ventilation, especially during a protective lung strategy in ARDS to prevent severe hypercapnia or counteract severe acidosis.
- Inspiratory flow rate (IFR): The inspiratory flow rate is usually set between 40 and 60 L/min to achieve an inspiratory and expiratory ratio of 1:2 or 1:3. A higher inspiratory flow rate (up to 90 L/min) is often recommended in cases of severe distal airway obstruction, such as acute COPD exacerbation or severe asthma exacerbation. This higher rate allows for longer expiratory time to empty the lungs, targeting an inspiratory-to-expiratory ratio (I:E) greater than 1:3.
- Fraction of inspired oxygen (FiO 2 ): FiO 2 should be adjusted to the minimum level necessary to maintain a pulse oximetry (SpO 2 ) reading of 90% to 96%. Avoiding hyperoxemia is crucial, as studies have demonstrated an increase in mortality among critically ill patients with excessive oxygen levels.
- Positive end-expiratory pressure (PEEP): PEEP increases the functional residual capacity and prevents the collapse of alveoli, thus reducing atelectrauma. Initially, the PEEP level is typically set at 5 cm H 2 O and adjusted based on the patient's underlying condition and oxygenation requirements. PEEP is titrated according to respiratory system mechanics or guidelines such as the ARDS network table in conditions such as ARDS.
- Trigger sensitivity: Triggers can be categorized into 2 types—flow trigger and pressure trigger. Pressure triggers are typically set at -2 cm H 2 O but should be avoided if auto-PEEP is suspected. In such cases, flow triggers should be used and set at a threshold of 2 L/min. [2] [11] [12] [13] [14] [15]
PAC mode: The following parameters must be adjusted on the ventilator when using PAC mode:
- Inspiratory pressure (Pi): As discussed above, the inspiratory pressure level is usually set between 10 and 20 cm H 2 O, based on the patient's underlying condition to achieve adequate V T .
- Inspiratory time (Ti): Inspiratory time is typically set to 1 second and adjusted to achieve an I:E ratio of 1:2 to 1:3.
- PEEP and FiO 2 settings are selected similarly to VAC mode. However, the inspiratory pressure adds additional pressure to the peak airway pressure and may further increase the risk of barotrauma.
SIMV/PSV mode: When SIMV/PSV mode is selected, the initial settings include the following:
- Pressure support (PS): The pressure support typically ranges from 5 to 15 cm H 2 O for spontaneous breaths initiated by the patient above the set rate. It can be adjusted as needed to maintain certain minute ventilation.
- Tidal volume (V T ): The tidal volume is set similarly to VAC mode to achieve targeted minute ventilation without causing ventilator-associated lung injury (typically 4 to 8 mL/kg PBW) for the non-spontaneous breaths.
Airway Pressure Release Ventilation Mode
APRV is a form of continuous positive airway pressure (CPAP) characterized by timed pressure release while allowing for spontaneous breathing (see Graph. APRV Pressure Cycles With Superimposed Spontaneous Breathing). [16] APRV provides continuous pressure to keep the lungs open, with a timed release to lower the set pressure. This continuous pressure phase facilitates the recruitment of proximal and distal alveoli by transmitting pressure to the chest wall. The prolonged continuous pressure phase, along with the short release phase, helps prevent continuous cycles of recruitment-derecruitment seen in pressure or volume control ventilation settings. This mechanism aids in avoiding atelectrauma, barotrauma, and ventilator-induced lung injury.
The timed release in APRV facilitates passive exhalation and enhances CO 2 clearance. Due to its reliance on spontaneous ventilation, APRV necessitates less sedation than conventional modes, reducing the risk of sedation-related complications (see Graph. Tidal Volume Comparison During APRV Versus Conventional Ventilation). Spontaneous breathing can increase end-expiratory lung volume, decrease atelectasis, and improve ventilation to dependent lung regions. Spontaneous breathing in APRV can raise end-expiratory lung volume, reduce atelectasis, and enhance ventilation in dependent lung regions. Moreover, spontaneous breathing improves the hemodynamic profile by lowering intrathoracic pressure and enhancing preload and cardiac output.
Setting up APRV involves adjusting 4 main variables: P-high, P-low, T-high, and T-low. [17] P-high represents the continuous pressure set, whereas P-low signifies the pressure release phase of the cycle. T-high determines the duration of the continuous pressure, whereas T-low indicates the release phase duration. Initially, the patient should be placed on assist control/volume control (AC/VC) immediately post-intubation until paralysis subsides. Subsequently, an inspiratory hold maneuver should be performed to determine the plateau pressure, which becomes the P-high and typically ranges from 27 to 29 cm H 2 O. However, obese patients may require higher pressures. P-low is usually set to 0, considering that in most cases, intrinsic PEEP (iPEEP) prevents full exhalation.
The T-high is typically set to 4 to 6 seconds, while the T-low is adjusted to 0.2 to 0.8 seconds in restrictive lung disease and 0.8 to 1.5 seconds in obstructive lung disease cases. Examining the flow-time waveform on the ventilator is crucial to set the T-low accurately. The T-low should ideally be around 75% of the peak expiratory flow rate (PEFR) for optimal ventilation (see Graph. Peak Expiratory Flow Rate Curve Illustration). [18] Continuous monitoring and readjustment of T-low to maintain this target are necessary as lung recruitment progresses. During APRV, FiO 2 levels should be titrated downward gradually as the patient's comfort and oxygenation permit.
Spontaneous breathing is paramount in APRV, and a slight amount of pressure support or automatic tube compensation should be provided to counter the endotracheal tube's intrinsic resistance. Hypoxemia in APRV can be addressed by increasing both P-high and T-high settings. [19] Alternatively, shortening the T-low can also help correct hypoxemia. APRV allows for permissive hypercapnia; however, excessive hypercapnia can be managed by reducing sedation or increasing P-high and T-high settings. Increasing T-low can also alleviate hypercapnia, but this approach is limited as APRV relies on iPEEP during P-low to maintain lung recruitment. Augmenting T-low may reduce iPEEP, thereby risking alveolar derecruitment.
- Issues of Concern
Ventilator-Associated Lung Injury
Ventilator-associated lung injury often occurs when ventilator settings are not adjusted based on PBW, especially in conditions like ARDS characterized by stiff lungs. Utilizing a lung-protective strategy involving low tidal volumes and targeted airway pressures is crucial to prevent lung injuries in these cases. [20]
Ventilator-Associated Events
Ventilator-associated events refer to a deterioration in respiratory status after a period of stability or improvement on the ventilator, accompanied by evidence of infection or inflammation and laboratory confirmation of respiratory infection. [21] Risk factors for ventilator-associated events include sedation (such as with benzodiazepines or propofol), fluid overload, high tidal-volume ventilation, and high inspiratory driving pressures. [22] Strategies to mitigate ventilator-associated events include implementing ventilator bundles to reduce sedation, conducting daily spontaneous awakening and breathing trials, promoting early mobilization, adopting conservative fluid and transfusion strategies, and employing lung-protective ventilation strategies. Recent studies have explored the impact of these interventions on patient outcomes, including the effectiveness of ventilator bundles. [23] [24]
Hemodynamic Changes
Transitioning a patient to mechanical ventilation shifts from natural negative pressure ventilation to positive pressure ventilation, impacting heart-lung physiology and altering hemodynamic status. Positive pressure ventilation elevates intrathoracic pressure, leading to decreased right ventricular preload and left ventricular preload and afterload. Additionally, the increased pressure augments the right ventricular afterload. [25] Although these effects may have minimal impact on the hemodynamics of a healthy individual, they can cause significant alterations in critically ill patients. For instance, a patient with acute pulmonary edema may benefit from reduced preload, whereas this change may not be beneficial for someone in septic shock.
- Clinical Significance
Clinical strategies for ventilator management may include lung-protective, obstructive, and intermediate strategies.
Lung-Protective Strategy
The lung-protective strategy is recommended for patients at risk of developing acute lung injury or progressing to ARDS. This approach involves using low tidal volume ventilation, as demonstrated in landmark trials such as the ARMA study, which showed improved mortality in ARDS patients with low tidal volume ventilation. This method is used to prevent barotrauma, volume trauma, and atelectatic trauma. Patients with conditions such as pneumonia, severe aspiration, pancreatitis, and sepsis are examples of those at risk and should be treated using the lung-protective strategy. A tidal volume (V T ) of 6 mL/kg based on ideal body weight is recommended. [26] [27] [28] In patients with acute lung injury progressing to ARDS, lung recruitment diminishes, and shunts develop, reducing functional lung volume. A low tidal volume strategy offsets the decreased functional lung volume. Tidal volume should not be adjusted based on minute ventilation goals; the respiratory rate should be adjusted based on minute ventilation goals and the patient's acid-base status. A starting respiratory rate of 16 breaths per minute is generally suitable for most patients to maintain normocapnia. [29]
A blood gas analysis should be obtained approximately 30 minutes after initiating mechanical ventilation, guiding adjustments to the respiratory rate based on the patient's acid-base status and PaCO 2 levels. If PaCO 2 significantly exceeds 40 mm Hg, the respiratory rate should be increased; conversely, if PaCO 2 is notably below 40 mm Hg, the respiratory rate should be decreased. End-tidal carbon dioxide (EtCO 2 ) is not a reliable indicator of PaCO 2 due to physiological shunt, dead space, and reduced cardiac output influencing EtCO 2 levels. The inspiratory flow rate is typically set at 60 L/min but can be adjusted higher if the patient demonstrates increased inspiratory effort at the onset of inspiration. [30] Following intubation, it is advisable to reduce the FiO2 to 40% immediately to prevent hyperoxemia. [13] The lung-protective strategy involves controlling both the FiO 2 and PEEP adjustments simultaneously. Poor oxygenation in acute lung injury results from derecruited alveoli and a physiological shunt. Consequently, FiO2 and PEEP should be incrementally increased together to address this issue. The oxygenation target should align with the ARDSnet protocol, aiming for 88% to 95% range.
ARDSnet PEEP/FiO 2 protocol: After initiating mechanical ventilation, it is essential to regularly reassess its effects on the patient, particularly on the alveoli. [31] This assessment involves monitoring the plateau pressure and driving pressure. The plateau pressure reflects the pressure exerted on small airways and alveoli and should ideally be maintained below 30 to avoid volume trauma and lung injury resulting from alveolar overdistension. An inspiratory pause must be initiated to measure the plateau pressure.
The driving pressure represents the tidal volume relative to lung compliance, indicating the "functional" lung volume that remains recruitable and unshunted. Calculating the driving pressure involves subtracting the PEEP level from the plateau pressure. [32] If the driving pressure exceeds 14, reducing tidal volume (V T ) to 4 mL/kg is crucial when plateau and driving pressures surpass these thresholds. Increasing the respiratory rate can help offset the decrease in minute ventilation, although permissive hypercapnia may be unavoidable. Permissive hypercapnia is a strategy that tolerates elevated PCO 2 levels to facilitate lung-protective ventilation with low tidal volumes. [33] However, recruitment maneuvers have been associated with increased mortality in moderate-to-severe ARDS and should not be used routinely. [34]
Obstructive Strategy
Patients with obstructive lung diseases like asthma and COPD are typically treated initially with noninvasive ventilation. However, in some cases, they may require intubation and mechanical ventilation. Obstructive lung diseases are characterized by narrowed airways and small airway collapse during expiration, leading to increased airflow resistance and decreased expiratory flow rates. This condition prolongs exhalation time, making it difficult to exhale the tidal volume before the next inhalation fully. As a result, residual air may remain in the chest at the start of inhalation. As the air becomes trapped in the alveoli, intrathoracic pressure increases, leading to a phenomenon known as auto-PEEP. This elevated pressure must be overcome during inhalation. As more air becomes trapped in the chest, flattening the diaphragm and expanding the lungs decrease compliance, leading to dynamic hyperinflation. With the progression of auto-PEEP and dynamic hyperinflation, there is increased work of breathing, reduced inhalation efficiency, and a risk of hemodynamic instability due to elevated intrathoracic pressure. Given these challenges, the ventilator strategy must counteract these pathologically increased pressures. Additionally, to address the obstructive process effectively, ventilatory management should be integrated with maximal medical therapy, including in-line nebulizers.
Extending the expiratory phase to allow for a complete exhalation can reduce auto-PEEP and dynamic hyperinflation when managing the ventilator for an obstructive patient. [2] Most patients require deep sedation to avoid over-breathing the ventilator and excessive inspiratory efforts. Tidal volume (V T ) should be set to 8 mL/kg, while the initial respiratory rate (RR) should be set at 10 breaths per minute. [30] These settings provide sufficient time for full expiration, thus reducing auto-PEEP. This approach often aligns with the permissive hypercapnia strategy, focusing on lower tidal volumes and adequate oxygenation rather than PaCO 2 levels. The inspiratory flow rate should be 60 L/min, while FiO 2 should be maintained at 40% after initiating ventilation. In obstructive lung diseases, the primary issue is ventilation rather than oxygenation, so increasing FiO 2 is generally unnecessary. Minimal PEEP is recommended, with some studies suggesting a PEEP of 0 and others suggesting a small amount to counteract auto-PEEP.
The ventilator waveform should be carefully assessed. If the waveform does not return to baseline (0) by the start of the next breath, the respiratory rate must be reduced to prevent increased hyperinflation and auto-PEEP. If a patient experiences a sudden desaturation or drop in blood pressure, they should be disconnected from the ventilator to allow for a complete exhalation. A clinician may assist exhalation by applying pressure to the patient's chest. A comprehensive evaluation, including ruling out pneumothorax due to volume trauma, is necessary. [29] If plateau pressures remain chronically high, it is also essential to rule out the possibility of pneumothorax.
Intermediate Strategy
The PReVENT trial found no significant difference between an intermediate tidal volume strategy (10 mL/kg) and a low tidal volume strategy (6 mL/kg) in patients without ARDS. [35] For patients without obstructive physiology or acute lung injury risk, an intermediate tidal volume strategy (8-10 mL/kg) may be appropriate. Typically, these patients do not experience significant oxygenation or ventilation challenges, so minimal ventilator settings are often adequate. Starting with a tidal volume (V T ) of 8 mL/kg, respiratory rate (RR) of 16, inspiratory flow rate (IFR) of 60 L/min, FiO 2 of 40%, and PEEP of 5 cm H 2 O, with titration as needed, is a reasonable starting point.
- Other Issues
Ventilator bundles are crucial in preventing ventilator-associated events. These measures include minimizing sedation, conducting daily spontaneous breathing trials, promoting early mobilization, adopting conservative fluid and transfusion strategies, and implementing lung-protective strategies.
Sedation is critical in treating patients on mechanical ventilation, especially in providing pain control and ensuring comfort post-intubation. An "analgesia first" sedation strategy is preferred, with fentanyl being a commonly used agent due to its minimally hypotension-inducing hemodynamic properties. [36] [37] If a patient remains agitated despite starting analgesic sedation, additional agents like propofol may be considered based on the patient's hemodynamic status and clinical requirements. In addition, obtaining a chest x-ray and blood gas analysis is crucial to confirm proper endotracheal tube placement and evaluate minute ventilation. Although some centers use ultrasound for endotracheal tube confirmation, it is not yet standard practice everywhere. Regular monitoring of plateau pressures is essential to evaluate alveolar integrity.
All patients undergoing mechanical ventilation should have the head of the bed elevated to at least 30°. Based on a 2016 Cochrane review on ventilator-associated pneumonia, a semi-recumbent position (30°-60°) reduced clinically suspected ventilator-associated pneumonia by 25.7% compared to a 0° to 10° supine position. Nonetheless, it is noteworthy that the available data on this topic is limited. [38]
Airway Maintenance
If a patient suddenly desaturates, clinicians should follow the DOPES mnemonic to identify potential causes. DOPES stands for displacement, obstruction of the endotracheal tube or airways, pneumothorax/pulmonary embolism/pulmonary edema, equipment failure, and stacked breaths. The patient should be disconnected from the ventilator and switched to a bag valve mask. The individual performing bagging should ventilate calmly and allow for a full exhalation. Following this, clinicians should adopt a systematic approach, as mentioned below.
- Is the patient's EtCO 2 still showing a good waveform? If not, the endotracheal tube may have been dislodged.
- Does the patient bag easily or with difficulty? If bagging is difficult, it may indicate obstructive problems such as an obstructed endotracheal tube, pneumothorax, or bronchospasm.
- Equipment failure is suggested if the patient bags easily and SpO 2 rises rapidly. During the failure evaluation, another clinician should assess the patient using an ultrasound of the lungs and heart and obtain a chest X-ray. If no other cause of desaturation is found, pulmonary embolism should be considered.
Venous Thromboembolism Prophylaxis
Invasive mechanical ventilation independently increases the risk of venous thromboembolism in the intensive care unit. [39] Therefore, implementing prophylactic measures is crucial to prevent additional morbidities.
Gastrointestinal Prophylaxis
Gastrointestinal bleeding affects approximately 5% of critically ill patients who are not receiving prophylaxis. [40] In a multicenter study, invasive mechanical ventilation was identified as 1 of the 2 independent risk factors for gastrointestinal bleeding (odds ratio for bleeding, 15.6; 95% CI, 3.0 to 80.1). [41] Therefore, implementing an acid suppression strategy for mechanically ventilated patients has been recommended. [42] Proton pump inhibitors are the most effective agents for preventing clinically important gastrointestinal bleeding, although their use may increase the risk of pneumonia. Continuing acid suppression beyond discharge from the intensive care unit is unnecessary. [43]
- Enhancing Healthcare Team Outcomes
Emphasizing the importance of effective communication among interprofessional healthcare team members is crucial when caring for mechanically ventilated patients. Respiratory therapists are critical in ventilator management, and their expertise should be effectively utilized. [44] Notably, assigning a single dedicated healthcare professional for ventilator management is crucial to ensure consistency and accuracy in care. To ensure proper treatment alignment, all adjustments to the ventilator must be communicated and coordinated with other healthcare team members responsible for the patient's overall care.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
APRV Pressure Cycles With Superimposed Spontaneous Breathing. APRV involves pressure cycles with superimposed spontaneous breathing, and this mode functions as a form of CPAP. Here, P-high represents the CPAP level, and T-high indicates the duration (more...)
Tidal Volume Comparison During APRV Versus Conventional Ventilation. In APRV, release volumes augment ventilation, which is associated with decreasing airway pressure and lung distension. Conversely, during conventional ventilation, tidal (more...)
Peak Expiratory Flow Rate Curve Illustration. A patient with initially low lung compliance exhibits a steeper expiratory flow curve (30° angle) and requires a short release phase (T-low) of 0.3 seconds (in this example) to terminate expiratory (more...)
Disclosure: Sean Hickey declares no relevant financial relationships with ineligible companies.
Disclosure: Abdulghani Sankari declares no relevant financial relationships with ineligible companies.
Disclosure: Al Giwa declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Hickey SM, Sankari A, Giwa AO. Mechanical Ventilation. [Updated 2024 Mar 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- ISCCM Guidelines for the Use of Non-invasive Ventilation in Acute Respiratory Failure in Adult ICUs. [Indian J Crit Care Med. 2020] ISCCM Guidelines for the Use of Non-invasive Ventilation in Acute Respiratory Failure in Adult ICUs. Chawla R, Dixit SB, Zirpe KG, Chaudhry D, Khilnani GC, Mehta Y, Khatib KI, Jagiasi BG, Chanchalani G, Mishra RC, et al. Indian J Crit Care Med. 2020 Jan; 24(Suppl 1):S61-S81.
- Ventilator Management. [StatPearls. 2024] Ventilator Management. Mora Carpio AL, Mora JI. StatPearls. 2024 Jan
- [Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)]. [Zhonghua Jie He He Hu Xi Za Zh...] [Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)]. Pulmonary Function and Clinical Respiratory Physiology Committee of Chinese Association of Chest Physicians, Chinese Thoracic Society, Pulmonary Function Group of Respiratory Branch of Chinese Geriatric Society. Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12; 47(2):101-119.
- Review [Hypercapnic respiratory failure. Pathophysiology, indications for mechanical ventilation and management]. [Med Klin Intensivmed Notfmed. ...] Review [Hypercapnic respiratory failure. Pathophysiology, indications for mechanical ventilation and management]. Kreppein U, Litterst P, Westhoff M. Med Klin Intensivmed Notfmed. 2016 Apr; 111(3):196-201. Epub 2016 Feb 22.
- Review Mechanical ventilation: invasive versus noninvasive. [Eur Respir J Suppl. 2003] Review Mechanical ventilation: invasive versus noninvasive. Brochard L. Eur Respir J Suppl. 2003 Nov; 47:31s-37s.
Recent Activity
- Mechanical Ventilation - StatPearls Mechanical Ventilation - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Find in topic
CALCULATORS
Related topics.
INTRODUCTION
There are several indications for the initiation of invasive mechanical ventilation in the intensive care unit (ICU) ( table 1 ). The common modes of ventilation, initial settings, and supportive care for intubated patients are discussed in this topic review. Intubation and complications of invasive mechanical ventilation are described separately. (See "Ventilator-induced lung injury" and "Clinical and physiologic complications of mechanical ventilation: Overview" and "Direct laryngoscopy and endotracheal intubation in adults" and "Rapid sequence intubation in adults for emergency medicine and critical care" and "The decision to intubate" and "Complications of the endotracheal tube following initial placement: Prevention and management in adult intensive care unit patients" .)
Invasive mechanical ventilation is defined as the delivery of positive pressure to the lungs via an endotracheal or tracheostomy tube.
During mechanical ventilation, a predetermined mixture of air (ie, oxygen and other gases) is forced into the central airways and then flows into the alveoli. As the lungs inflate, the intra-alveolar pressure increases. A termination signal (usually flow or pressure) eventually causes the ventilator to stop forcing air into the central airways and the central airway pressure decreases. Expiration follows passively, with air flowing from the higher-pressure alveoli to the lower pressure central airways.
Noninvasive ventilation (NIV) is delivered through an alternative interface, usually a face mask. Patient selection and indications for NIV are discussed in detail separately. (See "Noninvasive ventilation in adults with acute respiratory failure: Benefits and contraindications", section on 'Patients likely to benefit' .)
INDICATIONS
Invasive mechanical ventilation is most often used to fully or partially replace the functions of spontaneous breathing by performing the work of breathing and gas exchange in patients with respiratory failure ( table 1 and table 2 and table 3 ) [ 1 ]. Invasive mechanical ventilation may also be useful in those who require airway protection to reduce the risk of aspiration (eg, depressed mental status from an overdose, patients with variceal bleeding). Importantly, regardless of the indication, invasive mechanical ventilation should be considered early in the course of illness and should not be delayed until the need becomes emergent. (See "Mechanical ventilation of adults in the emergency department" and "Measures of oxygenation and mechanisms of hypoxemia" and "The evaluation, diagnosis, and treatment of the adult patient with acute hypercapnic respiratory failure" .)

IMAGES
COMMENTS
This state-of-the-art review provides an update on the basic physiology of respiratory mechanics, the working principles, and the main ventilatory settings, as well as the potential complications of mechanical ventilation.
Abstract. This review addresses how the combination of physiology, medicine and engineering principles contributed to the development and advancement of mechanical ventilation, emphasising the most urgent needs for improvement and the most promising directions of future development.
The article emphasizes the need for nurses to embrace evidence-based knowledge of how the various modes of ventilators operate, on strategies for managing patient responses to mechanical ventilator support, and causes of patient-ventilator dyssynchrony.
Identify key indications for invasive mechanical ventilation in patients with compromised airways, impaired ventilation, or hypoxemic respiratory failure. Implement safe and effective mechanical ventilation strategies tailored to individual patient needs and responses.
This chapter focuses on the components of a mechanical ventilator. In brief, a ventilator consists of a source of power, some mechanism to control the gas delivery and concentration, some monitors and sensors, and safety features which include filters and alarms.
A mechanical ventilator either fully or partially supports a patient’s work of breathing. The basic build of a mechanical ventilator can be described according to (1) input power, (2) source of pressurised gas, (3) drive mechanism, or (4) control circuit. Input power. Pneumatic: Compressed medical gases are used as the energy source.
Invasive mechanical ventilation is defined as the delivery of positive pressure to the lungs via an endotracheal or tracheostomy tube. During mechanical ventilation, a predetermined mixture of air (ie, oxygen and other gases) is forced into the central airways and then flows into the alveoli.
A mechanical ventilator is a machine that takes over the work of breathing when a person is not able to breathe enough on their own. The mechanical ventilator is also called a ventilator, respirator, or breathing machine.
Figure Block Diagram of a basic mechanical ventilator. A mechanical ventilator is an automatic machine, designed to provide all or part of the work the body must produce to move air (gas) from the inside to the outside and vice versa.
Mechanical ventilation is a type of therapy that helps you breathe or breathes for you when you can’t breathe on your own. You might be on a ventilator during surgery or if your lungs aren’t working properly. Mechanical ventilation keeps your airways open, delivers oxygen and removes carbon dioxide.