- Create account
- Contributions

Cell biology/Membrane Assembly: Signal Hypothesis
Here is the link to the ITunes U Lecture from Berkeley. Membrane Assembly: Signal Hypothesis
- mutate sequences to find one (or more) regions of genes required for import.
- "isolate" signal protein-fuse it to a reporter protein (like GFP) to see if the signal is sufficient.
- This sequence has 5 basic charges in a row, which is common for a signal protein.
- Ribosomes require protein subunits to travel to the nucleus so they must have a nuclear localization signal (NLS) .
- In the nucleus, the protein subunits are assembled in complex with the ribosomal RNA.
- Finally, the ribosomes must have another signal, nuclear export signal (NES) to get out of the nucleus
Nucleocytoplasmic Exchange
Or Mechanism of Import and Export of Proteins Into/From the Nucleus.

- Involves a several carrier proteins and a GTP binding (regulatory) protein. The GTP protein must be bound to the cargo that is imported or exported out of the nucleus.
- The central feature of this regulatory pathway can be reduced to the conversion of protein, called Ran, from a guanosine diphosphate (GDP) bound state to a guanosine triphosphate (GTP) bound state (GDP or GDP are bound to Ran) or the reverse reaction.
- There are many small GDP/GTP binding proteins that regulate the traffic of proteins and cytoskeletal events throughout the cell.
- This small regulatory protein, Ran, is important because it controls the entry and exit of all proteins that must enter/exit the nucleus.
- Ran-GEF (always in the nucleus) binds in the nucleus to Ran-GDP and displaces the GDP allowing GTP to replaces it.
- Ran-GAP (always in the cytoplasm) binds Ran-GTP and stimulates GTP hydrolysis, so a phosphate group drops off, creating GDP.
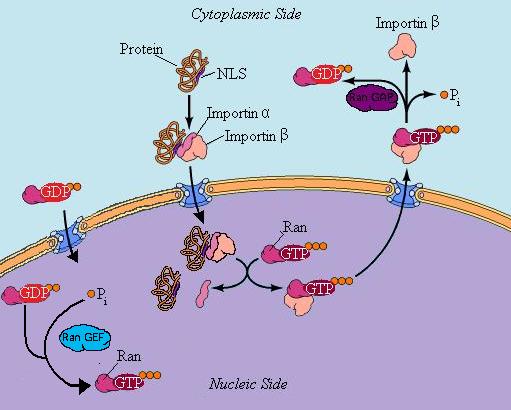
What happens when a protein (cargo), in the cytoplasm wants to be imported into the nucleus?
See Diagram above.
- The cargo protein has an NLS (nuclear localization signal) and alpha importin binds to the NLS.
- Beta importin binds to the alpha importin.
- Beta importin is responsible for driving the protein complex just created through the pore. It makes contact with the inside of the pore complex (mildly hydrophobic), and binds and dissociates with protein sequences of the pore.
- Ran-GDP has already diffused in.
- Ran-GEF acts on (activates) Ran-GDP converting it to Ran-GTP.
- Ran-GTP binds with the alpha beta cargo complex and causes it to dissociate.
- The cargo molecule does whatever it was supposed to to (i.e. transcription or ribosome production).
- Ran-GTP binds to the beta importin (alpha importin follows) and the complex travels through the nuclear pore.
- On the cytoplasmic side the Ran-GAP pulls off a phosphate group, from Ran-GTP creating Ran-GDP
- Cells have lots of importin alphas which are devoted to importing a dozen (or two) cargo molecules.
What happens when a protein (cargo)wants to be exported from the nucleus?
- Exportin binds to NES (nuclear export signal) on the cargo protein.
- Exportin is responsible for interacting with the nuclear pore.
- When the complex exits the pore, Ran-GAP removes a phosphate group from Ran-GTP, creating Ran-GDP.
- Ran-GDP then detaches from the complex leaving the cargo protein in the cytoplasm along with exportin.
- Exportin must be transported back into the nucleus with Ran-GDP.
*Cells have lots of exportin alphas which are devoted to importing a dozen (or two) cargo molecules.
- Regulation of import/export of certain molecules is managed by regulating importin and exportin proteins.
- The NES or NLS is exposed late in the assembly of the desired protein/complex (ribosome) and so their is no attachment point for the transportation enzymes until the cargo molecules are ready for import/export.
Secretory or Membrane Protein Transport
Proteins that are transported to the into or out of cell membrane are very different from nucleocytoplasm transport. The pores that are on the cell membrane are quite small (diameter = 8 angstroms ) and only allow proteins to pass as they are being synthesized (compared to the nuclear membrane that allows all small proteins to pass and larger proteins to pass with Ran-GDP or Ran-GTP).
Enter the Endoplasmic Reticulum
The endoplasmic reticulum is a conveyor belt wrapped within the cell on which ribosomes are deposited. In pancreatic cells, 90% of the ribosomes are touching the cytoplasmic surface of the ER. Protected in the interior of the ER is a clear space called the lumen. The transfer of secretory proteins (as they are being made by the ribosomes) from the membrane to the lumen determines their future as a secretory protein. When the proteins complete production, the ER fuses with the cell membrane and releases the proteins to the outside of the cell.
- In the 1940s it first became possible to prepare cells and tissues to study with the electron microscope.
George Palade confirmed the function of the endoplasmic reticulum and Golgi Apparatus for producing secretory proteins. He also found that ribosomes are responsible for protein manufacturing. He studied the movement of proteins within the pancreas (90% of their energy is devoted to making secretory proteins and their endoplasmic reticulum (ER) has 25X the surface area of the plasma membrane).
- Palade performed an experiment where he homogenized pancreatic tissue and created vesicles that have ribosomes still bound (with their messenger RNA) and show that the ribosomes are caught in the act of translating a nascent polypeptide chain.
- He then took these vesicles and added translation factors, ATP, and GTP , transfer RNA and other materials necessary for protein synthesis. The vesicles would then create full proteins from their nascent state inside the vesicle.
- How do you prove that the proteins are discharged into the interior of the vesicle via vectorial translocation?

- Vectorial translocation is the name of transfer in a unidirectional manner.
- To prove this you can use an earlier study (protease protection). For this study, you would take the vesicles, ATP, GTP, transfer RNA and radiolabeled amino acids. Then treat one group with trypsin (protease) and another with trypsin and a detergent. This would show that trypsin can only act upon the amino acids when the detergent is used.
- From this we know that we can create a protein in a test tube.
How do these frequently polar molecules cross the lipid bilayer membrane?
- Cesar Milstein won the Nobel prize for monoclonal antibodies. But we are more interested in his work with the antibody light chain because he discovered the N-terminal extension on an immature protein, antibody light chain.
- Earlier work with SDS gels gave the mature light chain's structure and weight of 23.5kD.
- It is possible to get ribosomes and translation necessities, without a membrane, by breaking open reticulocytes.
- He found that the in vitro created protein, a precursor protein, had a weight of 25.5kD.
- So there are an extra 20 amino acids at the N-terminus of precursor secretory proteins that is later cleaved before the protein becomes fully developed.
- Günter Blobel (Palade's protege) also won the Nobel Prize.
- His study involved stripping ER vesicles of their ribosomes and adding mRNA and ribosomes to create fully formed proteins (without their N-terminus extension) inside of the ER vesicle.
To be continued in the next lecture.
Please feel free to add details or make changes where necessary. Contact me via email if you need help. Thanks, April
Previous Lecture
Next Lecture
Return Home
- Cell biology
- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now

Synthesis of Membrane Proteins and the "Signal Hypothesis"
ADVERTISEMENTS:
All of a cell’s proteins are synthesized by ribosomes, including, of course, those proteins that are destined for inclusion in the plasma membrane.
Cytoplasmic ribosomes in eukaryotic cells occur in two states: (1) “attached” (ribosomes associated with the membranes of the endoplasmic reticulum) and (2) “free” (ribosomes freely dispersed in the cytosol). Both attached and free ribosomes are believed to contribute proteins to the plasma membrane.
Synthesis of Membrane Proteins and the “Signal Hypothesis”:
Principally as a result of the work of G. Blobel, D. D. Sabatini, C. M. Redman, C. Milstein, J. E. Rothman, J. Lenard, and H. F. Lodish, the mechanism that routes newly synthesized proteins to their proper destinations in the cell has gradually unfolded. A major contribution to this end has been the confirmation of the signal hypothesis proposed in the early 1970s by Blobel and Sabatini.
According to this hypothesis (Fig. 15-19), proteins that are to be either (a) secreted from the cell, (b) dispatched to lysosomes, or (c) incorporated into the plasma membrane or membranes of the endoplasmic reticulum are encoded by mRNA molecules that contain a special nucleotide sequence called a “signal.”
The signal segment encodes a chain of about 16 to 26 amino acids that appears at or near the beginning of the polypeptide chain. Near its N- terminus, the signal sequence contains polar, basic residues, whereas the central domain is apolar. When a ribosome attaches to the mRNA in the cytosol and begins to translate the message, the signal sequence or signal peptide emerging from the ribosome is recognized by a ribonucleoprotein complex in the cytosol called the signal recognition particle (i.e., SRP).
SRP, which consists of a 7 S cytoplasmic RNA molecule and six polypeptides, binds to the signal sequence, bringing about a temporary halt to protein synthesis by that ribosome. Synthesis is resumed only if the SRP-ribosome complex attaches to the endoplasmic reticulum; ribosomes synthesizing polypeptides that lack a signal sequence do not interact with SRP and do not attach to the endoplasmic reticulum.
The amino acid sequences of a number of signal peptides have now been determined. Although they contain a specific distribution of hydrophobic and charged residues, no primary sequence homologies appear to exist. Consequently, it is believed that SRP must recognize certain features contained in the signal peptide’s secondary and tertiary structure.
SRP-ribosome complexes attach to the endoplasmic reticulum at specific sites occupied by SRP receptors (also called docking proteins). Once “docking” is completed, the SRP-ribosome-docking protein interaction is replaced by a functional ribosome- membrane junction and the synthesis of the polypeptide encoded by the mRNA is resumed. SRP returns to the cytosol where it can participate in another round of signal recognition and docking (i.e., the “SRP cycle” of Fig. 15-19a).
When protein synthesis is resumed by the docked ribosome, the elongating polypeptide chain passes through the ER membrane into the intracisternal space. This process, termed “translocation,” is presumed to involve active participation of elements of the membrane. Translocation of the growing polypeptide into the intracisternal space is depicted in Figure 15-19 as taking place through a pore-like opening in the membrane solely to indicate that the membrane’s permeability barrier is transiently altered.
However, it is not known with certainty whether translocation through the membrane takes place through such a proteinaceous water-filled channel or directly through the lipid bilayer. In most instances, the signal sequence is eventually cleaved from the remainder of the growing polypeptide by an extrinsic enzyme called signal peptidase attached to the lumenal surface of the ER.
If the protein being synthesized is destined for secretion from the cell, completion of synthesis is followed by the protein’s release from the ribosome into the intracisternal space. The ribosome then detaches from the membrane and the mRNA and may participate in another round of protein synthesis. At the same time, the permeability barrier of the ER is restored.
Proteins discharged into the ER cisternae in this manner are ultimately conveyed to the Golgi apparatus for chemical modification prior to secretion. In the case of proteins destined to be regular constituents of the endoplasmic reticulum or the plasma membrane, translocation into the intracisternal space is aborted before synthesis is finished so that the polypeptide is left anchored in the membrane (Fig. 15-19b). The information halting the translocation is likely contained in the polypeptide itself and is recognized by the translocation apparatus.
For peripheral membrane proteins facing the exterior of the cell or the lumenal phase of the ER, the signal sequence is followed by synthesis of the hydro- philic portion of the polypeptide. If an integral membrane protein is being synthesized, the hydro- philic portion is followed by a hydrophobic segment that remains anchored in the lipid bilayer. For proteins that span the membrane, synthesis is completed with the production of a final hydrophilic segment that faces the cytosol.
By comparing parts a and b of Figure 15-19, it is seen that the major distinction between the synthesis of secretory proteins and membrane proteins is that secretory proteins are released into the lumenal phase of the ER, whereas membrane proteins remain anchored in the ER.

Addition of sugars to presumptive plasma membrane glycoproteins may occur soon after the hydrophilic portions of the molecules enter the ER cisternae. The membrane glycoprotein then migrates from the ER to the Golgi apparatus. Although the mechanism for this transfer is still uncertain, it is believed to take place either by dispatchment of small vesicles from the ER, which then migrate to and fuse with the Golgi membranes, or by lateral “flow” along the ER membranes to the Golgi. Glycosylation of the membrane proteins is completed in the Golgi apparatus.
The Golgi apparatus dispatches completed plasma membrane glycoproteins as small vesicles that migrate to and fuse with the plasma membrane. The overall process is summarized in Figure 15-20, which also shows that the intracellular/extracellular orientation of the membrane protein is maintained throughout its passage from the ER to the plasma membrane.

Some integral proteins have hydrophilic parts that face the interior of the cell and some peripheral proteins are associated only with the membrane’s cytoplasmic face. Although a similar mechanism is not precluded for the synthesis of these membrane proteins, they could be synthesized by free ribosomes.
Following release of these proteins in the cytosol they may diffuse to the plasma membrane. Integral proteins would be spontaneously inserted into the membrane by a hydrophobic segment (Fig. 15- 20), whereas peripheral proteins would attach to the membrane through polar interactions. Peripheral proteins reaching the plasma membrane in this manner could not pass through the membrane to the exterior surface because they could not traverse the hydrophobic membrane core.
Prokaryotic cells do not contain an endoplasmic reticulum. Secretory proteins and new plasma membrane proteins are synthesized by ribosomes that attach to the inner surface of the plasma membrane. As in eukaryotic cells, ribosome attachment follows synthesis of a signal peptide encoded in the protein’s mRNA. The protein is then dispatched through the cell membrane and into the extracellular space.
Synthesis of Membrane Lipids:
In eukaryotic cells, phospholipid synthesis is associated with the endoplasmic reticulum, whereas in prokaryotic cells lipid synthesis is a property of the cytoplasmic half of the plasma membrane. It is therefore likely that lipid synthesis in eukaryotic cells takes place in the cytoplasmic half of the ER membranes.
Following synthesis, the phospholipid becomes at least temporarily part of the interior monolayer but may either be enzymatically translocated to the outer layer or flip-flop between the two layers. In view of the fact that outer monolayer lipids are derived from the inner monolayer, an absolute asymmetry is precluded.
Because in prokaryotic cells lipid synthesis occurs in the plasma membrane, incorporation into the membrane’s structure is direct. In eukaryotic cells, however, presumptive plasma membrane lipid must make its way from the ER to the plasma membrane.
This is believed to occur by one or both of two processes. Newly synthesized lipids inserted into ER membranes may make their way to the plasma membrane by the same mechanism that translocates ER membrane proteins (Fig. 15-20); that is both membrane proteins and lipids pass from the ER to the Golgi apparatus and are later dispatched to the plasma membrane via small vesicles.
The cytosol of eukaryotic cells contains a number of phospholipid transport proteins that function to transfer phospholipid molecules from one cellular membrane to another. These transport proteins might also play a major role in mediating the passage of phospholipids from the membranes of the ER to the plasma membrane.
Related Articles:
- Role of Ribosomes in Protein Synthesis | Genetics
- Difference between Smooth and Rough Endoplasmic Reticulum | Cytoplasm
- Anybody can ask a question
- Anybody can answer
- The best answers are voted up and rise to the top
Forum Categories
- Animal Kingdom
- Biodiversity
- Biological Classification
- Biology An Introduction 11
- Biology An Introduction
- Biology in Human Welfare 175
- Biomolecules
- Biotechnology 43
- Body Fluids and Circulation
- Breathing and Exchange of Gases
- Cell- Structure and Function
- Chemical Coordination
- Digestion and Absorption
- Diversity in the Living World 125
- Environmental Issues
- Excretory System
- Flowering Plants
- Food Production
- Genetics and Evolution 110
- Human Health and Diseases
- Human Physiology 242
- Human Reproduction
- Immune System
- Living World
- Locomotion and Movement
- Microbes in Human Welfare
- Mineral Nutrition
- Molecualr Basis of Inheritance
- Neural Coordination
- Organisms and Population
- Photosynthesis
- Plant Growth and Development
- Plant Kingdom
- Plant Physiology 261
- Principles and Processes
- Principles of Inheritance and Variation
- Reproduction 245
- Reproduction in Animals
- Reproduction in Flowering Plants
- Reproduction in Organisms
- Reproductive Health
- Respiration
- Structural Organisation in Animals
- Transport in Plants
- Trending 14
Privacy Overview

Signal hypothesis
The signal hypothesis relates to mechanisms such as the biological transport of proteins in cells to the appropriate organelles by insertion into the membranes or secreted outside the cell. Newly synthesized proteins are endowed with an intrinsic signal that must be deciphered at the target sites. Günter Blobel suggested, in 1975, that this signal determines their ability to go to and through the endoplasmic reticulum membrane, and he called this theory the signal hypothesis .
The signal consists of a hydrophobic peptide, the signal peptide , made up of about 20 amino acids in a particular order, which are the first to appear when the polypeptide chain is being synthesized. This peptide seems to signal to the cell that the protein to which it is bound must be transported, since when the peptide binds to a cytoplasmic protein this causes the protein to be secreted. It seems that the hydrophobicity of the signal peptide is one of the most important factors for protein transport to occur. The molecular principles described by Blobel that form the basis of this process are universal. signal peptides have a certain charge, since they have lys or arg in their coding sequences, the positive side, marked by these amino acids, always faces the cytosol. In addition, the translocation will be determined by this charge and the cost for the cell to carry out the translocation will also depend on it, since the charge must always be facing the cytosolic side, many times the protein chain is longer on the side where it is located. the negative charge of the signal peptide, which would imply a greater work to move this long chain through the translocon (membrane protein that fulfills the function of translocating the protein.
Contenido relacionado
Solenostemon
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 April 2011
Spatial expression of the genome: the signal hypothesis at forty
- Karl S. Matlin 1
Nature Reviews Molecular Cell Biology volume 12 , pages 333–340 ( 2011 ) Cite this article
2840 Accesses
18 Citations
6 Altmetric
Metrics details
- Cell signalling
- Gene expression
The signal hypothesis, formulated by Günter Blobel and David Sabatini in 1971, and elaborated by Blobel and his colleagues between 1975 and 1980, fundamentally expanded our view of cells by introducing the concept of topogenic signals. Cells were no longer just morphological entities with compartmentalized biochemical functions; they were now active participants in the creation and perpetuation of their own form and identity, the decoders of linear genetic information into three dimensions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Blobel, G. & Sabatini, D. D. in Biomembranes Vol. 2 (ed. Manson, L. A.) 193–195 (Plenum Publishing Corporation, New York, 1971).
Book Google Scholar
Blobel, G. & Dobberstein, B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67 , 835–851 (1975).
Article CAS Google Scholar
Blobel, G. & Dobberstein, B. Transfer of protein across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J. Cell Biol. 67 , 852–862 (1975).
Devillers-Thiery, A., Kindt, T., Scheele, G. & Blobel, G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc. Natl Acad. Sci. USA 72 , 5016–5020 (1975).
Blobel, G. Intracellular protein topogenesis. Proc. Natl Acad. Sci. USA 77 , 1496–1500 (1980).
Dobberstein, B., Blobel, G. & Chua, N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii . Proc. Natl Acad. Sci. USA 74 , 1082–1085 (1977).
Jackson, R. C. & Blobel, G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc. Natl Acad. Sci. USA 74 , 5598–5602 (1977).
Katz, F. N., Rothman, J. E., Lingappa, V. R., Blobel, G. & Lodish, H. F. Membrane assembly in vitro : synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc. Natl Acad. Sci. USA 74 , 3278–3282 (1977).
Goldman, B. M. & Blobel, G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc. Natl Acad. Sci. USA 75 , 5066–5070 (1978).
Erickson, A. H. & Blobel, G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J. Biol. Chem. 254 , 11771–11774 (1979).
CAS PubMed Google Scholar
Maccecchini, M. L., Rudin, Y., Blobel, G. & Schatz, G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc. Natl Acad. Sci. USA 76 , 343–347 (1979).
Ahmed, S. et al. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nature Cell Biol. 12 , 111–118 (2010).
Bechtel, W. Discovering Cell Mechanisms: The Creation of Modern Cell Biology (Cambridge Univ. Press, Cambridge, UK, 2006).
Google Scholar
Carnoy, J. B. La Biologie Cellulaire: Étude Comparée De La Cellule Dans Les Deux Règnes (J. Van In, Paris, 1884).
Porter, K. R., Claude, A. & Fullam, E. F. A study of tissue culture cells by electron microscopy: methods and preliminary observations. J. Exp. Med. 81 , 233–246 (1945).
Palade, G. E. & Porter, K. R. Studies on the endoplasmic reticulum. I. Its identification in cells in situ . J. Exp. Med. 100 , 641–656 (1954).
Palade, G. E. Studies on the endoplasmic reticulum. II. Simple dispositions in cells in situ . J. Biophys. Biochem. Cytol. 1 , 567–582 (1955).
Palade, G. E. A small particulate component of the cytoplasm. J. Biophys. Biochem. Cytol. 1 , 59–68 (1955).
Rheinberger, H.-J. Toward a History of Epistemic Things (Stanford Univ. Press, California, 1997).
Siekevitz, P. & Palade, G. E. A cytochemical study on the pancreas of the guinea pig. V. In vivo incorporation of leucine-1-C 14 into the chymotrypsinogen of various cell fractions. J. Biophys. Biochem. Cytol. 7 , 619–630 (1960).
Gazzinelli, G. & Dickman, S. R. Incorporation of amino acids into protein by beef-pancreas ribosomes. Biochim. Biophys. Acta 61 , 980–982 (1962).
Redman, C. M., Siekevitz, P. & Palade, G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J. Biol. Chem. 241 , 1150–1158 (1966).
Redman, C. M. & Sabatini, D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc. Natl Acad. Sci. USA 56 , 608–615 (1966).
Sabatini, D. D., Tashiro, Y. & Palade, G. E. On the attachment of ribosomes to microsomal membranes. J. Mol. Biol. 19 , 503–524 (1966).
Sabatini, D. D. In awe of subcellular complexity: 50 years of trespassing boundaries within the cell. Annu. Rev. Cell Dev. Biol. 21 , 1–33 (2005).
Harrison, T. M. Messenger Ribonucleic Acid and Polysomes in a Mouse Plasmacytoma . Thesis, Univ. Cambridge (1972).
Milstein, C., Brownlee, G. G., Harrison, T. M. & Mathews, M. B. A possible precursor of immunoglobulin light chains. Nature New Biol. 239 , 117–120 (1972).
Blobel, G. & Sabatini, D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J. Cell Biol. 45 , 130–145 (1970).
Sabatini, D. D. & Blobel, G. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J. Cell Biol. 45 , 146–157 (1970).
Lingappa, V. R., Devillers-Thiery, A. & Blobel, G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc. Natl Acad. Sci. USA 74 , 2432–2436 (1977).
Shields, D. & Blobel, G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc. Natl Acad. Sci. USA 74 , 2059–2063 (1977).
Chang, C. N., Blobel, G. & Model, P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc. Natl Acad. Sci. USA 75 , 361–365 (1978).
Walter, P., Jackson, R. C., Marcus, M. M., Lingappa, V. R. & Blobel, G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc. Natl Acad. Sci. USA 76 , 1795–1799 (1979).
Walter, P. & Blobel, G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 77 , 7112–7116 (1980).
Walter, P. & Blobel, G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J. Cell Biol. 91 , 557–561 (1981).
Walter, P. & Blobel, G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J. Cell Biol. 91 , 551–556 (1981).
Walter, P., Ibrahimi, I. & Blobel, G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro -assembled polysomes synthesizing secretory protein. J. Cell Biol. 91 , 545–550 (1981).
Gilmore, R., Blobel, G. & Walter, P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J. Cell Biol. 95 , 463–469 (1982).
Gilmore, R., Walter, P. & Blobel, G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J. Cell Biol. 95 , 470–477 (1982).
Meyer, D. I., Krause, E. & Dobberstein, B. Secretory protein translocation across membranes-the role of the 'docking protein'. Nature 297 , 647–650 (1982).
Meyer, D. I., Louvard, D. & Dobberstein, B. Characterization of molecules involved in protein translocation using a specific antibody. J. Cell Biol. 92 , 579–583 (1982).
Walter, P. & Blobel, G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299 , 691–698 (1982).
Walter, P. & Blobel, G. Subcellular distribution of signal recognition particle and 7SL-RNA determined with polypeptide-specific antibodies and complementary DNA probe. J. Cell Biol. 97 , 1693–1699 (1983).
Walter, P. & Blobel, G. Disassembly and reconstitution of signal recognition particle. Cell 34 , 525–533 (1983).
Evans, E. A., Gilmore, R. & Blobel, G. Purification of microsomal signal peptidase as a complex. Proc. Natl Acad. Sci. USA 83 , 581–585 (1986).
Schatz, G. & Dobberstein, B. Common principles of protein translocation across membranes. Science 271 , 1519–1526 (1996).
Harmey, M. A., Hallermayer, G., Korb, H. & Neupert, W. Transport of cytoplasmically synthesized proteins into the mitochondria in a cell free system from Neurospora crassa . Eur. J. Biochem. 81 , 533–544 (1977).
Chua, N. H. & Schmidt, G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc. Natl Acad. Sci. USA 75 , 6110–6114 (1978).
Highfield, P. E. & Ellis, R. J. Synthesis and transport of the small subunit of chloroplast ribulose bisphosphate carboxylase. Nature 271 , 420–424 (1978).
Randall, L. L., Hardy, S. J. & Josefsson, L. G. Precursors of three exported proteins in Escherichia coli . Proc. Natl Acad. Sci. USA 75 , 1209–1212 (1978).
Ito, K., Mandel, G. & Wickner, W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc. Natl Acad. Sci. USA 76 , 1199–1203 (1979).
Date, T. & Wickner, W. T. Procoat, the precursor of M13 coat protein, inserts post-translationally into the membrane of cells infected by wild-type virus. J. Virol. 37 , 1087–1089 (1981).
CAS PubMed PubMed Central Google Scholar
Josefsson, L. G. & Randall, L. L. Processing in vivo of precursor maltose-binding protein in Escherichia coli occurs post-translationally as well as co-translationally. J. Biol. Chem. 256 , 2504–2507 (1981).
Date, T., Goodman, J. M. & Wickner, W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc. Natl Acad. Sci. USA 77 , 4669–4673 (1980).
Deshaies, R. J. & Schekman, R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J. Cell Biol. 105 , 633–645 (1987).
Simon, S. M. & Blobel, G. A protein-conducting channel in the endoplasmic reticulum. Cell 65 , 371–380 (1991).
Gorlich, D., Prehn, S., Hartmann, E., Kalies, K. U. & Rapoport, T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell 71 , 489–503, (1992).
Crowley, K. S., Liao, S., Worrell, V. E., Reinhart, G. D. & Johnson, A. E. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell 78 , 461–471 (1994).
Gorlich, D. & Rapoport, T. A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75 , 615–630 (1993).
Nakai, K. Protein sorting signals and prediction of subcellular localization. Adv. Protein Chem. 54 , 277–344 (2000).
Nakai, K. & Horton, P. Computational prediction of subcellular localization. Methods Mol. Biol. 390 , 429–466 (2007).
Kerr, S. C. & Corbett, A. H. Should INO stay or should INO go: a DNA “zip code” mediates gene retention at the nuclear pore. Mol. Cell 40 , 3–5 (2010).
Rothman, J. E. & Lodish, H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature 269 , 775–780 (1977).
Goldman, B. M. & Blobel, G. In vitro biosynthesis, core glycosylation, and membrane integration of opsin. J. Cell Biol. 90 , 236–242 (1981).
Audigier, Y., Friedlander, M. & Blobel, G. Multiple topogenic sequences in bovine opsin. Proc. Natl Acad. Sci. USA 84 , 5783–5787 (1987).
Kevles, D. J. & Hood, L. The Code of Codes: Scientific and Social Issues of the Human Genome Project (Harvard Univ. Press, Massachusetts,1992).
Inouye, H. & Beckwith, J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro . Proc. Natl Acad. Sci. USA 74 , 1440–1444 (1977).
Silhavy, T. J., Shuman, H. A., Beckwith, J. & Schwartz, M. Use of gene fusions to study outer membrane protein localization in Escherichia coli . Proc. Natl Acad. Sci. USA 74 , 5411–5415 (1977).
Emr, S. D., Hanley-Way, S. & Silhavy, T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23 , 79–88 (1981).
Kalderon, D., Roberts, B. L., Richardson, W. D. & Smith, A. E. A short amino acid sequence able to specify nuclear location. Cell 39 , 499–509 (1984).
Friedlander, M. & Blobel, G. Bovine opsin has more than one signal sequence. Nature 318 , 338–343 (1985).
Wiedmann, M., Kurzchalia, T. V., Bielka, H. & Rapoport, T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J. Cell Biol. 104 , 201–208 (1987).
Gould, S. J., Keller, G. A., Hosken, N., Wilkinson, J. & Subramani, S. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108 , 1657–1664 (1989).
Casanova, J. E., Apodaca, G. & Mostov, K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell 66 , 65–75 (1991).
Beckmann, R. et al. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science 278 , 2123–2126 (1997).
von Heijne, G. Models for transmembrane translocation of proteins. Biochem. Soc. Symp. 46 , 259–273 (1981).
CAS Google Scholar
Van den Berg, B. et al. X-ray structure of a protein-conducting channel. Nature 427 , 36–44 (2004).
Becker, T. et al. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science 326 , 1369–1373 (2009).
Rapoport, T. A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membrane. Nature 450 , 663–669 (2007).
Download references
Acknowledgements
The author is grateful for support of this project from the US National Library of Medicine and the US National Institutes of Health (NIH), to W. Green, N. Matlin, A. Engelberg and J. Collier for review of the manuscript, and to members of the Committee on Conceptual and Historical Studies of Science at the University of Chicago, Illinois, USA, for stimulating discussions. Photographs in figure 2 were provided by the Laboratory of Molecular Biology at the University of Cambridge, UK, and by N. Dwyer, NIH, USA. The author thanks the many individuals who have provided information for this article through interviews.
Author information
Authors and affiliations.
Karl S. Matlin is at Department of Surgery, The University of Chicago, 5841 South Maryland Avenue, MC 5032, SBRI J557, Chicago, Illinois 60637-1470, USA. [email protected],
Karl S. Matlin
You can also search for this author in PubMed Google Scholar
Ethics declarations
Competing interests.
The author declares no competing financial interests.
Related links
Further information.
Karl S. Matlin's homepage
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Matlin, K. Spatial expression of the genome: the signal hypothesis at forty. Nat Rev Mol Cell Biol 12 , 333–340 (2011). https://doi.org/10.1038/nrm3105
Download citation
Published : 13 April 2011
Issue Date : May 2011
DOI : https://doi.org/10.1038/nrm3105
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
The steps of the signal hypothesis that emerged from the experiments of Blobel and his colleagues are illustrated below. Recall that the synthesis of any protein starts with assembly of a translation initiation complex, followed by polypeptide elongation. During elongation, the growing polypeptide moves through and emerges from a channel, or ...
signal hypothesis A hypothesis to explain how ribosomes become attached to membranes within cells in order to deliver the appropriate proteins to cell organelles, such as mitochondria and chloroplasts, or transport proteins outside the cell membrane.It proposes that the leading end of the nascent polypeptide chain consists of a signal peptide.This sticks out from the ribosome and is recognized ...
Steps below refer to handout. See Becker fig. 22-16 (20-16) or Purves 12.15 . 1. What is the Signal Hypothesis? Ribosome unattached to ER starts making protein. (step 1) If nascent (growing, incomplete) peptide has a "signal peptide," then ribosome plus growing chain will attach to ER membrane, and growing chain will enter ER as it grows. 2.
So when the protein is folded, the 2 regions are side by side and provide the signal for import. The T antigen sequence required for entry is: proline-lysine-lysine-lysine-arginine-lysine-valine. This sequence has 5 basic charges in a row, which is common for a signal protein. Ribosomes:
"The signal hypothesis" ... Based on elegant biochemical experiments, Blobel described in 1975 the various steps in these processes. The signal consists of a peptide, i.e. a sequence of amino acids in a particular order that form an integral part of the protein. He also suggested that the protein traverses the membrane of the endoplasmic ...
According to this hypothesis (Fig. 15-19), proteins that are to be either (a) secreted from the cell, (b) dispatched to lysosomes, or (c) incorporated into the plasma membrane or membranes of the endoplasmic reticulum are encoded by mRNA molecules that contain a special nucleotide sequence called a "signal."
•According to this hypothesis , proteins which are to be either 1) secreted from the cell , 2) dispatched to lysosomes 3) incorporated into the plasma membrane or 4) membranes of E.R , are encoded by m-RNA molecules which contain a special nucleotide sequence called the signal at their 5' end . •This signal encodes a chain of about 16-26
The signal hypothesis relates to mechanisms such as the biological transport of proteins in cells to the appropriate organelles by insertion into the membranes or secreted outside the cell. Newly synthesized proteins are endowed with an intrinsic signal that must be deciphered at the target sites. Günter Blobel suggested, in 1975, that this signal determines their ability to go to and through ...
Consistent with the signal hypothesis is the fact that the information contained within 321 signal peptides is interpreted by the sequen- tial interaction of specific proteins. Once a nascent protein has traversed the mem- brane, it is known that another component postulated in the signal hypothesis, the signal peptidase, removes the signal se ...
The signal hypothesis, formulated by Günter Blobel and David Sabatini in 1971, and elaborated by Blobel and his colleagues between 1975 and 1980, fundamentally expanded our view of cells by ...